Contact: Allan Chen (510) 486-4210, [email protected]

Tests of a military prototype field version of the laser ablation-based explosives detection system at the Yuma Proving grounds in 2008. The detector was able to discriminate with 85 percent accuracy whether more than 100 samples contained residues of several types of explosives from between 30 to 50 meters (90 to 150 feet) away, or whether the composition was of materials such as rock, wood, metal, plastics.
A soldier in a humvee aims a portable device at an abandoned car 50 meters away (more than 150 feet). Pressing a button, a laser in the device fires. She reads a screen, and beckons her patrol to move away quickly.
In a lab, a technician is inserting a fragment of a toy into a sample case, inserting it into a machine, and pressing a button. He inserts one fragment after another—each test takes only a few seconds. The paint on some of the toy fragments are testing positive for lead.
Another technician, this time at an abandoned industrial facility, is collecting samples of concrete, metal and building materials to bring back to an analysis lab. He’s part of a team looking for beryllium contamination. Beryllium is a light metal—number four on the periodic table—that is extremely poisonous to living things. He’ll collect hundreds of samples in just a few days. Analyzing each one will require less than a minute.
None of these scenes is science fiction anymore. And each one is an example of green chemistry—chemical analysis that is quick and results in no chemical waste generation. The military application can save the lives of soldiers in combat. The other applications can keep our children safe from toys and materials illegally coated with lead paints, and workers from suffering the effects of chemical poisoning.
The key is a technology developed by scientists at the Department of Energy’s Lawrence Berkeley National Laboratory using laser ablation—aiming a laser beam at the material sample to be tested, causing a tiny amount of it to be vaporized so that spectroscopic (optical and mass) analysis techniques and unique computer software can analyze the sample in seconds.
Rick Russo, a scientist in Berkeley Lab’s Environmental Energy Technologies Division began studying the physics of laser ablation 28 years ago. “I was interested in the fundamental physics of what happens when you fire a laser at a solid sample, and ablate the material into a vapor. There’s so much physics involved that it’s phenomenal,” he says.
More than 200 publications, nine patents, and an R&D 100 award later, Russo and his research group are the pioneers of a new chemical analysis technology based on laser ablation. The two most common approaches to laser ablation chemical analysis are LIBS, (laser induced breakdown spectroscopy) in which the light from a tiny plasma is directly measured and related to the chemical element and its concentration, and LA-ICP-MS (laser ablation inductively coupled plasma mass spectrometry), in which tiny particles from the ablation are measured. On average, LIBS provides part-per-million sensitivity whereas LA-ICP-MS provides parts-per-billion sensitivity. Both approaches allow the measurement of the entire chemical composition of a sample target using a single laser pulse. Although he never entered his field to create a practical application, Russo realized that LA-ICP-MS and LIBS have many possible uses in the world.
In 2004, he created a company, with the help of Small Business Innovation Research grants, called Applied Spectra to bring the technology, and its many potential life-saving applications, to the marketplace. It sold its first LIBS device in 2008. The company’s corporate offices are in Fremont, California, a community 30 miles south of Berkeley, in the eastern part of Silicon Valley. It also has a small manufacturing facility in Aberdeen, Maryland. The jobs created by the company are “green” jobs, since the technology is a waste reducing one.
“Most of the samples in the world that we want to analyze are solid,” says Russo. “The conventional method of analysis requires an entire analysis infrastructure that is based on dissolving these solids in strong acid so that the resulting liquid can be analyzed using standard methods. This is very dirty, generates a lot of chemical waste, and requires a lot of labor and time.”
In contrast, laser ablation requires no acid dissolution and so generates no hazardous wastes. “Laser ablation is green technology,” says Russo. “Besides eliminating chemical waste, it saves energy. My goal is to change the paradigm for the way that chemical analysis is done.”
Laser ablation is very fast, allowing technicians to conduct more tests on samples in real time. And, laser ablation testing is cheaper—only a technician is needed to operate the tool. The technician’s job is to place the sample and operate the machine; each test takes less than 30 seconds. No PhDs are needed.
Remote military explosives detection
Russo’s basic research has been funded for many years by the U.S. Department of Energy’s Office of Basic Energy Science. But his application research for laser ablation has been funded by many other offices, such as the Nuclear Non-Proliferation and Security Agency of DOE, which was interested in laser ablation’s potential for quick identification of nuclear wastes at nuclear manufacturing sites, and the Department of Defense, which was looking for a way to remotely and quickly identify explosive residues that might provide tell-tale hints of car bombs and other terrorist weapons.
Russo and his co-workers tested a military prototype field version of the LIBS system at the Yuma Proving grounds in 2008. The detector was able to discriminate with 85 percent accuracy whether more than 100 samples contained residues of several types of explosives from between 30 to 50 meters (90 to 150 feet) away, or whether the composition was of materials such as rock, wood, metal, plastics or, in one case, food materials—salami and cheese.
Applied Spectra has developed several commercial versions of this tool, and is planning to release a hand-held, and transportable version later in 2009.
Detecting lead and other heavy metals
With so many news stories recently about imported toys and other products painted or otherwise contaminated by lead, testing labs and consumer safety authorities need faster, accurate ways of determining which products to be concerned about.
Many European and Asian nations have adopted regulations requiring the removal of hazardous materials from new and to-be-recycled electronic components called Restrictions on Hazardous Substances (RoHS) and Waste Electrical and Electronic Equipment (WEEE). More stringent U.S. regulations are also being looked at. Lead, cadmium, mercury, hexavalent chromium, and organic materials PBB and PBDE are among the dangerous materials that these regulations are aiming to remove from the products we use every day. Research has demonstrated that LIBS testing is more accurate than today’s X-ray fluorescence technology for quick screening of these materials.
Laser ablation-based testing can also be used in a variety of other “green” and “green policing” applications. For example, one manufacturer of electronic components is using it to check on the soldering of electronic components to ensure that they are free of lead, which many countries now require. It can also analyze the purity of silicon used in solar photovoltaic panels for quality control.
Assisting with detection of wastes at contaminated sites
Those performing the clean-up of old mining sites will find LIBS technology useful in identifying the contaminants at the sites, information that can guide waste management experts determining the best methods to use to clean up and isolate what’s present.
Russo has been discussing the possible use of LA-ICP-MS and LIBS methods to assist in hazardous waste reduction programs at several of the Department of Energy’s National Laboratories, including the Idaho National Laboratory, and the Lawrence Livermore National Laboratory. In Livermore, program managers are conducting about 4,000 to 5,000 tests for beryllium contamination per year, although more than 90 percent of those tests turn up negative. The testing takes time, and generates its own hazardous waste. LA testing would require less than 30 seconds per test, far less than what the current test requires, and would generate no waste.
Some of the research also has been oriented toward using LA to detect residues of nuclear weapons development for use in nuclear non-proliferation programs.
What happens during laser ablation
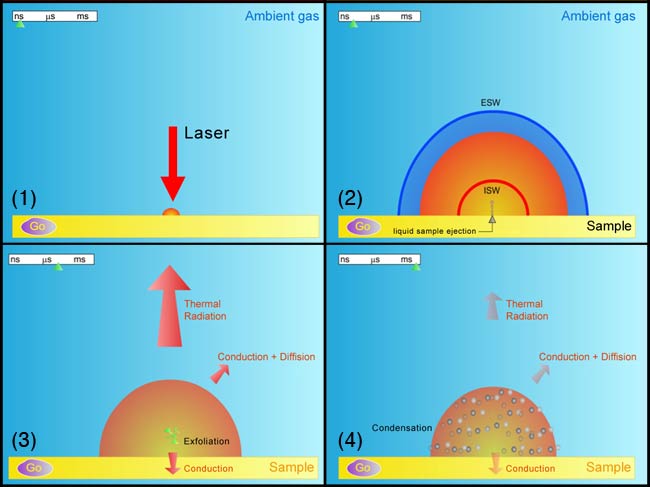
At the start of the laser pulse until one nanosecond later, violent evaporation takes place at the material’s surface. From one nanosecond to one microsecond (1,000 ns) after the end of the pulse, the high-temperature plume expands outward. From one microsecond to 1,000 microseconds (1 millisecond) after the end of the pulse, the heat leaks away through radiative heating and the plume cools.
Thanks to years of study, Russo’s group now has time resolved images, analyses of ablated material, and a mathematical model of the laser ablation process. At the start of the laser pulse until one nanosecond later, violent evaporation takes place at the material’s surface (see figure 1). From one nanosecond to one microsecond (1,000 ns) after the end of the pulse, the high-temperature plume expands outward. From one microsecond to 1,000 microseconds (1 millisecond) after the end of the pulse, the heat leaks away through radiative heating and the plume cools. At the end of this interval, about one millisecond after the end of the pulse, the vapor plume drops to the boiling temperature of the sample, and condenses back to its solid form—albeit as a tiny dusting of particles (nanoparticles). The formation of nanoparticles in these laser ablation plumes was the basis of Smalley’s discovery of buckeyballs from graphite.
In LIBS, the plasma emission from the ablated sample is gathered using special optics. A spectrometer analyzes the white light emitted from the plasma, separating the light into its colors (wavelengths). Every sample has a unique spectral signature thanks to its chemical components. An ICCD (intensified charge coupled device) camera records the signature, converting the light into pixels of information.
A variation on LIBS, called LA-ICP MS (laser ablation inductively coupled plasma mass spectrometry), the ablated material itself, in the form of fine particles, can be gathered by a stream of a carrier gas such as argon, and heated to a high temperature in order to be converted to a plasma (atoms stripped of their electrons). The chemical composition of the plasma is then analyzed using a mass spectrometer.
LIBS testing is extremely sensitive. It can detect materials at concentrations of the tens of parts per million. LA-ICP MS is even more sensitive, capable of detecting chemicals at the parts-per-billion level.
Russo says that “We understand the fundamental physics of it, and we better understand which parameters to use to make the most effective measurements with laser ablation.” His group’s research serves as an example of fundamental research to better understand a natural phenomenon that led to an unexpected, but useful application in the industrial and commercial world.
Most of the funding for the basic science research has come from DOE’s Office of Basic Energy Science, and DOE’s National Nuclear Security Administration. Funding for Applied Spectra has come from the Department of Defense SBIR program.
More information:
- Laser Spectroscopy and Applied Materials Group Environmental Energy Technologies Division
http://teamd.lbl.gov/
- Applied Spectra: http://www.appliedspectra.com/