Contact: Dan Krotz, (510) 486-4019
The red and blue images appear ghostly, like a fleeting glimpse of something that’s never been seen before — which is true. Using computer simulations, Berkeley Lab scientists have developed the first predicted images of water molecules surrounding a nanoparticle, in this case an iron-oxide mineral called hematite.
The simulations indicate that the size and shape of the nanosized mineral determines the way in which water molecules layer around it. And this influences how the mineral interacts with its environment, including other nanoparticles, dissolved ions, and the surfaces of larger minerals and bacteria.
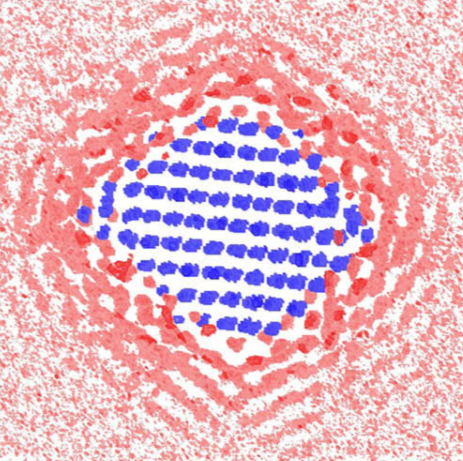
Why nanosized minerals do what they do: This computer simulation reveals the cross section of the water density around a 2.7 nanometer faceted particle. The blue indicates an iron site, pink indicates the area with low water density, and red indicates the area with high water density.
The images are a peek into the hidden world of nanosized minerals, which are important components of geochemical cycles in soils, groundwater, rivers and lakes. They’re also key players in some of the biggest challenges facing scientists today. Cleaning up contaminants left over from abandoned mines, or learning how to store carbon underground — where it can’t contribute to climate change — will require a better understanding of how nanosized minerals participate in these processes.
Addressing these headline-grabbing problems is one of the reasons behind the recently created Berkeley Nanogeoscience Center, located at Berkeley Lab, which seeks to uncover the roles played by nanosized particles in geochemical processes — both manmade and natural. The multidisciplinary group of scientists utilizes cutting edge imaging technologies and computer simulations to learn what makes nanosized minerals tick.
Consider subsurface contaminants. In California, decades of mining activity have yielded large quantities of toxic metal ions that threaten to leach into watersheds. These ions are often adsorbed onto mineral nanoparticles.
“To understand how such contaminants move, we have to understand how nanoparticles move through the subsurface, carrying with them metal ions that are sorbed onto their surface,” says Jill Banfield, a principal investigator in the Geochemistry Department of Berkeley Lab’s Earth Sciences Division, and a UC Berkeley professor in the Department of Earth and Planetary Sciences and in the Department of Environmental Science, Policy and Management.
There’s one problem, however. Nanosized minerals abide by their own, often poorly understood rules. At the nanoscale, which is smaller than 100 nanometers in diameter (one nanometer is one-billionth of a meter), a mineral is more surface than volume. And this can change the way it reacts in unexpected ways.
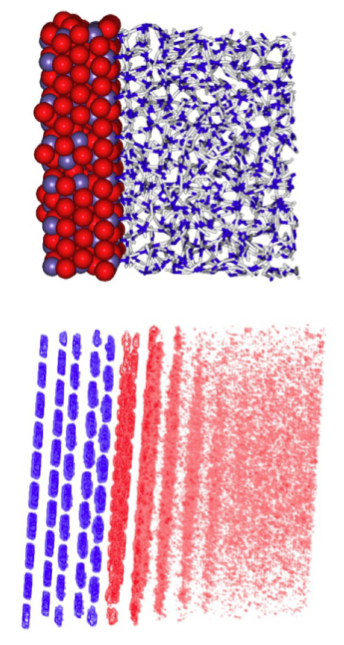
The top image is a representation of a computer simulation of a hematite particle's two dimensional surface. The bottom image is the computer simulation, where the blue indicates the iron sites in the solid, dark red indicates high water density, and pink indicates low water density.
To explore this world, scientists at the Berkeley Nanogeoscience Center utilize transmission electron microscopy at Berkeley Lab’s National Center for Electron Microscopy, which offers extremely high-resolution imaging. Berkeley Lab’s Advanced Light Source, a national user facility that generates intense light for scientific research, is used to characterize the chemistry of nanoparticles and image their association with biopolymers and cells.
In their most recent work, the scientists used a dedicated computing cluster that’s tailored for nanogeoscience research. Dino Spagnoli, working with a team of scientists from Berkeley Lab’s Earth Sciences Division, performed molecular dynamics simulations of different shapes and sizes of a hematite nanoparticle to investigate how water molecules surround it.
“Based on the shape and size of the nanoparticle, and how water surrounds it, we can predict how ions will adsorb to the surface, which is essential to understanding crystal growth,” says Spagnoli, who is now with the Curtin University of Technology in Australia.
The simulations predict that water molecules enshroud nanoparticles in ordered layers that change their organization with particle size and shape. With larger faceted nanoparticles, water molecules at the corners are less layered. This makes it easier for an ion to swim to the nanoparticle’s surface. In contrast, water molecules become trapped around spherical nanoparticles, decreasing ion mobility.
“It is much easier for compounds to get to the surface of a faceted rather than a spherical particle,” adds Banfield. “Overall, we found that water behaves differently based on size and shape of the nanoparticle, and this influences how it reacts with other minerals.”
“Prediction of the effects of size and morphology on the structure of water around hematite nanoparticles” by Dino Spagnoli, Benjamin Gilbert, Glenn Waychunas, and Jillian Banfield appears in a recent issue of the journal Geochimica et Cosmochimica Acta. The paper was featured in the Editor’s Choice section of the May 22, 2009 issue of Science. This research was funded by the Department of Energy.
Additional Information:
- Learn more about the Berkeley Nanogeoscience Center