With several new models of electric vehicles hitting the market this year and more next year, President Obama’s goal of putting 1 million EVs on U.S. roads by 2015 is tantalizingly within grasp. But what will it take for that number to reach 10 million or even 100 million in 20 years?
The answer: batteries need significant improvements. Specifically, they need to be cheaper, safer, last longer and have higher energy. The battery research team at Lawrence Berkeley National Laboratory (Berkeley Lab), recognized as one of the best in the country, is engaged in high-risk, high-reward research in each of those four areas, striving for technology breakthroughs as well as incremental advances. Their work could help drive a transformation of the vehicle industry and make EVs as common as laptops and cell phones for American consumers.
“I think with incremental improvements in batteries, engineering advances in the car and support from the government, these are all things that will make it a reality,” says Berkeley Lab scientist Marca Doeff. “And there’s considerable enthusiasm among the population as a whole, so I think it’s going to happen.”

Some members of Berkeley Lab's battery research team (Photos by Roy Kaltschmidt, Berkeley Lab Public Affairs)
Indeed, it is a boom time for batteries. In the last three years, the battery group at Berkeley Lab has hired 24 researchers, and the budget for the Department of Energy’s Batteries for Advanced Transportation Technologies (BATT) program, which is managed by Berkeley Lab, has grown from $5 million four years ago to $16 million this year. More recently, Berkeley Lab’s battery team was part of two multimillion-dollar awards from DOE’s Advanced Research Projects Agency-Energy (ARPA-E) funded by Recovery Act money. In one, the Lab is working with Applied Materials, Inc. of Santa Clara, California, which was awarded $4.4 million to develop ultra-high energy, low-cost lithium-ion batteries using a novel manufacturing process. In the second, the Lab is working with Sion Power Corp. of Tucson, Arizona, which received $5 million to develop high-energy lithium-sulfur batteries for electric vehicles.
“The government has given billions of dollars [in low-interest loans and grants], venture capitalists are throwing money, and look at the number of battery startups in the last few years. It’s gone from two or three to dozens,” says Venkat Srinivasan, Berkeley Lab battery scientist and Acting Group Leader of the Electrochemical Technologies Group. “The great thing about the boom is there will be a lot of innovation.”
Battery Design: The Art of Trade-offs
Still, no one expects a smooth road to 100 million EVs. Batteries are complex electrochemical systems with some processes that even scientists don’t completely understand. The wanted chemical reactions are accompanied by unwanted side reactions that need to be controlled. The line between a powerful, stable battery and a powerful, unstable battery is often a thin one.
“On the one hand, you’d like to drive your car for 300 miles on a single charge. On the other hand, you have to realize you’re sitting on a high-density energy source,” says scientist Robert Kostecki, who has worked on batteries for 15 years and is also deputy director of Berkeley Lab’s Environmental Energy Technologies Division. “The more energy you pack in a small volume or small mass, the more hazardous behavior you can expect.”
Making a battery is all about trade-offs. Srinivasan uses a spider chart (see diagram below; for technical version click here) to show how present-day lithium-ion batteries compare to the DOE goals for the FreedomCAR, a plug-in hybrid electric vehicle (PHEV) with a range of 40 miles and a life of 15 years. “It’s like a string,” says Srinivasan. “You pull on one end, you’re going to do something on the other end.” For example, to increase the energy density, typically the life of the battery decreases, or the battery can be made safer, but then its energy density will be lower.
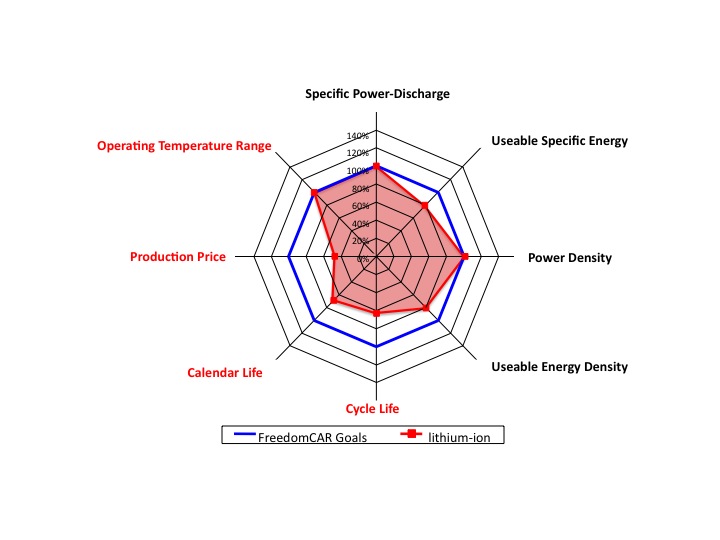
This "spider chart" compares the present-day performance of lithium-ion batteries with the goals of the FreedomCAR, which include 15-year life and 5,000 recharge cycles. The energy is associated with a car's range and the power with its acceleration. Safety, another important issue, is not included in this plot. (Chart courtesy Venkat Srinivasan and Vince Battaglia)
At Berkeley Lab, the focus is on lithium-ion batteries, which were first commercialized in 1991 and are still considered the best near-term option for transportation use. A “lithium-ion” battery, in fact, can refer to any of a variety of different chemistries, and the Berkeley Lab battery team is exploring a number of them. Which one will be the eventual winner is not clear yet, and there may not be a single winner because different applications have different requirements. While newer alternatives such as lithium-sulfur and lithium-air hold great promise, they will require technology breakthroughs before becoming a reality.
Part of the motivation to jump-start battery innovation is to bring battery manufacturing back to the United States. Production of lithium-ion batteries, mostly for cell phones and other portable electronics, moved to Asia, especially China, Japan and South Korea, nearly 20 years ago. “China and Japan have spent 15 years gaining knowledge in the art of making a battery. How do you beat that?” Srinivasan says. “You have to think of a scientific way to approach this problem.”
The Key to Extending Life
In principle, batteries are composed of a positively charged cathode, a negatively charged anode, and an electrolyte solution that carries charged ions between the two. When batteries fail, they can do so for any number of reasons. Broadly, the causes fall into two categories—mechanical degradation and chemical degradation. “It’s very hard to predict battery failure. We can’t simulate it,” Srinivasan says. “Berkeley is trying to get to a battery simulator by getting to the fundamentals of how batteries fail.
The Berkeley team is also taking a fundamental scientific approach to the chemical degradation by studying the protective layer that forms at the interface between the electrode and electrolyte—the solid electrolyte interface, or SEI. The SEI is one of the key components that enable function of a Li-ion battery.
Stabilizing the electrode/electrolyte interface has been pinpointed as critical to extending the life of a battery. The SEI inhibits spontaneous decomposition of the electrolyte—usually at the anode. “Unfortunately, we don’t fully understand how this layer forms and functions and what it is made of,” Kostecki says. “It still escapes our best instrumental techniques and experimental methodologies.”
While batteries for cell phones and personal electronics are not expected to operate much longer than two years, batteries for cars need to last at least 10 if not 15 years. “It’s not a simple engineering extrapolation to extend life from two years to 15 years,” says Kostecki. “It’s a tremendous challenge. You have to reduce the extent of the detrimental side effects in batteries by orders of magnitude.”
The SEI is a primary focus of research for Berkeley Lab battery scientists. The team brings to the problem its strength in diagnostics and modeling to detect and understand what is happening at the micro-, nano- and molecular levels as the SEI forms, identify the critical processes, then link those to the overall performance of the battery.
Cutting Costs
Another requirement to getting a significant number of electric vehicles on the road is cheaper batteries. Today’s lithium-ion batteries cost about $1,000/kilowatt-hour. The DOE’s goal is to bring that down to $150/kWh, which assumes a battery for an all-electric vehicle that can replace what most people drive today, meaning a range of close to 300 miles. “It’s going to be very difficult to reach that goal,” Doeff acknowledges, then adds, “It’s true we need to get the cost down, but I don’t know if we need to get it down that far.”
Depending on whether the battery is for an all-electric vehicle, a PHEV or an HEV (hybrid electric vehicle, such as most Toyota Priuses on the road today, which can go only a couple miles on its battery), the requirements would be different. Doeff and Tom Richardson work mainly on finding suitable materials for the cathode, one of the most expensive parts of a battery, along with the separator and electrolyte solution.
The most common cathode material in lithium-ion batteries is lithium cobalt oxide. However, cobalt can be very expensive, and also tends to come from countries that are not politically stable. “The long and short of it is we have to get rid of cobalt to lower the prices,” Doeff says.
Other cathode materials being looked at include lithium iron phosphate, which is attractive because it delivers a good amount of power and iron is inexpensive, but its energy density is inferior. It’s currently used in power tools and is one of the top choices for hybrids and PHEVs where power (acceleration) is of more concern than energy (range). The challenge is to get more energy out of it.
Another option is lithium manganese oxide spinel, advantageous because manganese is inexpensive, although it too has lower energy density. Doeff is also looking at titanium and aluminum as substitutes for cobalt.
The raw materials account for about 60 percent of a battery’s cost. The remaining 40 percent goes to the manufacturing, a complex process that can involve as many as 50 to 60 steps. Reducing manufacturing costs will require fundamental innovations in the way batteries are made. It is an area ripe for change as the battery manufacturing process has not evolved much since the voltaic pile was invented 210 years ago.
“We have materials scientists developing twenty-first century science. But if you look at the way batteries are manufactured today, it’s not much different from the original design that [Alessandro] Volta used in the nineteenth century,” Kostecki says. “That discrepancy between the innovation of state-of-the-art electrode materials and simplistic manufacturing methodologies is one of the limiting factors for lithium-ion batteries today. Manufacturing procedures currently are largely based on trial and error. Consequently, electrode material properties are seriously compromised by poor battery electrode design.”
For example, graphite is the state-of-the-art material used in the anodes of the vast majority of lithium-ion batteries. Lithium ions can travel in graphite only between the graphene layers, but they cannot move across this layered structure. Similarly, the electricity is only conducted within the plane of the layers. However, graphitic carbons for Li-ion battery applications have not been engineered to fully exploit these properties.
“The empirical way it’s done today is that battery companies contact the graphite manufacturer, try all forms of graphite available on the market, and then choose and optimize a selected few,” says Kostecki. “Using a more rational approach to design graphite’s structure would make electrodes perform better. I believe that materials scientists who work on the next generation of electrode materials should work in unison with engineers who can rationally design the battery electrodes and cells, rather than separate these two functions, as they are now. It’s an opportunity for Berkeley Lab to combine all of our resources and approach this problem in a coordinated, holistic way.”
Making Sure Batteries Stay Safe
Another important priority for Berkeley Lab battery researchers is safety, which has been an issue in laptops and other consumer devices. “Lithium batteries do go up in flames occasionally,” says Richardson. “It’s pretty rare, but once they burn, it’s hard to get them to stop. And there are issues of toxicity.”
It is precisely the advantages of lithium batteries—small in size and high in energy—that make them potentially dangerous. Several factors could cause a lithium battery to explode, including overcharging, manufacturing defects and physical changes to the battery. Although the odds of a single battery erupting are very small, an electric vehicle is likely to have hundreds of them in a series, with current running through each one. If the capacity of one cell is smaller than the others, it will get overcharged, leading possibly to thermal runaway. To deal with this, either the current could be diverted around the cell, which would add weight and volume, or the array could be designed so that it would just stop charging, which would limit the range.
Berkeley Lab is developing an internal self-actuating overcharge protection that would not significantly increase the weight or volume of the cell nor the complexity of the manufacturing. To do that, Richardson and Guoying Chen are looking at electroactive polymers, a class of polymers with unique properties. “The polymer will get oxidized when the cell is being overcharged and go from electrically insulating to conducting,” Chen explains. “So it generates a short in the cell, between the anode and the cathode, meaning no net current goes to the electrode and thus prevents the cell from being overcharged.”
So far, they have demonstrated that the concept works well with different polymers as well as different cathodes and anodes. “And it’s reversible too, so when you stop overcharging, the polymer goes back to being resistive,” Richardson adds.
The work now is focused on finding a polymer and a configuration that will give optimal performance. “How you put the polymer on the separator has a large effect, and also where you put it,” Chen says.
Wringing More Energy out of Lithium-Ion Batteries
On the flip side of higher safety is higher energy density, which means greater range for the vehicle. Within 10 years after lithium-ion batteries were commercialized in the early 1990s, their energy density doubled. Srinivasan believes it can double again within another decade.
There are three ways to get higher energy density: increase the capacity, increase the voltage or decrease the amount of inactive material in the battery. The team at Berkeley is involved with all three aspects. Materials research is being undertaken to find the next-generation high-capacity cathode and anode materials, and new electrolytes that allow the battery to operate at higher voltages without any detrimental side reactions. For example, the Berkeley team has started research with their partners in BATT to enable the use of a high voltage, stable cathode that promises to increase the energy density compared to the state of the art. In addition, the team has been pursuing avenues to decrease the amount of inactive material in the battery while maintaining the power capability and cycle life.
Assuming success with innovative materials and processes, the timeline from the lab to the marketplace is a long one for batteries. “People will tell you it takes 10 years and $100 million to develop a battery system,” says Doeff. “Even if we went into the lab next week and discovered the next big thing that had everything we needed, it would still take 10 years to develop. These are seemingly simple devices, but there’s so much we’re asking of them.”
Additional Information:
- BATT Program at Berkeley Lab
- Venkat Srinivasan’s This Week in Batteries blog
- Department of Energy’s Vehicle Technologies Program
