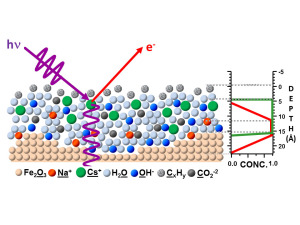
By utilizing X-ray standing waves to excite photoelectrons, SWAPPS delivers vital information about all the chemical elements at the heterogeneous interfaces found in batteries, fuel cells and other devices.
Researchers working at the Advanced Light Source (ALS) of the U.S. Department of Energy (DOE)’s Lawrence Berkeley National Laboratory (Berkeley Lab) have combined key features of two highly acclaimed X-ray spectroscopy techniques into a new technique that offers sub-nanometer resolution of every chemical element to be found at heterogeneous interfaces, such as those in batteries and fuel cells. This new technique is called SWAPPS for Standing Wave Ambient Pressure Photoelectron Spectroscopy, and it combines standing-wave photoelectron spectroscopy (SWPS) with high ambient pressure photoelectron spectroscopy (APPS).
“SWAPPS enables us to study a host of surface chemical processes under realistic pressure conditions and for systems related to energy production, such as electrochemical cells, batteries, fuel cells and photovoltaic cells, as well as in catalysis and environmental science,” says Charles Fadley, a physicist who holds joint appointments with Berkeley Lab’s Materials Sciences Division and the University of California Davis, where he is a Distinguished Professor of Physics. “SWAPPS provides all the advantages of the widely used technique of X-ray photoelectron spectroscopy, including element and chemical-state sensitivity, and quantitative analysis of relative concentrations of all species present. However with SWAPPS we don’t require the usual ultrahigh vacuum, which means we can measure the interfaces between volatile liquids and solids.”
Fadley is one of three corresponding authors of a paper describing SWAPPS in Nature Communications. The paper is titled “Concentration and chemical-state profiles at heterogeneous interfaces with sub-nm accuracy from standing-wave ambient-pressure photoemission.” The other two corresponding authors are Hendrik Bluhm, with Berkeley Lab’s Chemical Sciences Division, a pioneer in the development of APPS, and Slavomír Nemšák, now with Germany’s Jülich Peter Grünberg Institute. (See below for the complete list of authors).
(From left) Chuck Fadley, Ioannis Zegkinoglou, Slavomir Nemsak, Osman Karslioglu, Andrey Shavorskiy and Hendrik Bluhm at Beamline 11.0.2 of the Advanced Light Source (photo by Roy Kaltschmidt)
In terms of energies and wavelengths, X-rays serve as excellent probes of chemical processes. In the alphabet soup of X-ray analytical techniques, two in particular stand out for the study of chemistry at the interface where layers of two different materials or phases of matter meet. The first is SWPS, developed at the ALS by Fadley and his research group, which made it possible for the first time to selectively study buried interfaces in a sample with either soft or hard X-rays. The second is APPS, also developed at the ALS by a team that included Bluhm, which made it possible for the first time to use X-ray photoelectron spectroscopy under pressures and humidities similar to those encountered in natural or practical environments.
“Heterogeneous processes at solid/gas, liquid/gas and solid/liquid interfaces are ubiquitous in modern devices and technologies but often difficult to study quantitatively,” Bluhm says. “Full characterization requires measuring the depth profiles of chemical composition and state with enhanced sensitivity in narrow interfacial regions at the nanometer scale. By combining features of SWPS and APPS techniques, we can use SWAPPS to measure the elemental and chemical composition of heterogeneous interfaces with sub-nanometer resolution in the direction perpendicular to the interface.”
Says Fadley, “We believe SWAPPS will deliver vital information about the structure and chemistry of liquid/vapor and liquid/solid interfaces, in particular the electrical double layer whose structure is critical to the operation of batteries, fuel cells and all of electrochemistry, but which is still not understood at a microscopic level.”
Fadley, Bluhm, Nemšák and their collaborators used their SWAPPS technique to study a model system in which a nanometer layer of an aqueous electrolyte of sodium hydroxide and cesium hydroxide was grown on an iron oxide (hematite) solid. The spatial distributions of the electrolyte ions and the carbon contaminants across the solid/liquid and liquid/gas interfaces were directly probed and absolute concentrations of the chemical species were determined. The observation of binding-energy shifts with depth provided additional information on the bonding and/or depth-dependent potentials in the system.
“We determined that the sodium ions are located close to the iron oxide/solution interface, while cesium ions are on average not in direct contact with the solid/liquid interface,” Bluhm says. “We also discovered that there are two different kinds of carbon species, one hydrophobic, which is located exclusively in a thin film at the liquid/vapor interface, and a hydrophilic carbonate or carboxyl that is evenly distributed throughout the liquid film.”
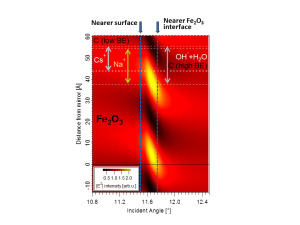
SWAPPS measures the depth profiles of chemical elements with sub-nanometer resolution in the direction perpendicular to the interface utilizing an X-ray standing wave field that can be tailored to focus on specific depths, i.e., near the surface or near the iron oxide interface.
A key to the success of this study was the use of X-ray standing waves to excite the photoelectrons. A standing wave is a vibrational pattern created when two waves of identical wavelength interfere with one another: one is the incident X-ray and the other is the X-ray reflected by a mirror. Interactions between standing waves and core-level electrons reveal much about the depth distributions of each chemical species in a sample.
“Tailoring the X-ray wave field into a standing wave can be used to achieve greater depth sensitivity in photoelectron spectroscopy,” Fadley says. “Our combination of an oscillatory standing-wave field and the exponential decay of the photoelectron signal at each interface gives us unprecedented depth resolution.”
In their Nature Communications paper, the authors say that future time-resolved SWAPPS studies using free-electron laser or high-harmonic generation light sources would also permit, via pump-probe methods, looking at the timescales of processes at interfaces on the femtosecond time scale.
“The range of future applications and measurement scenarios for SWAPPS is enormous,” Fadley says.
This work was carried out at ALS Beamline 11.0.2, which is operated by Berkeley Lab’s Chemical Sciences Division and hosts two ambient-pressure photoemission spectroscopy endstations.
In addition to Fadley, Bluhm and Nemšák, other authors of the Nature Communications paper describing SWAPPS were Andrey Shavorskiy, Osman Karslioglu, Ioannis Zegkinoglou, Peter Greene, Edward Burks, Arunothai Rattanachata, Catherine Conlon, Armela Keqi, Farhad Salmassi, Eric Gullikson, See-Hun Yang and Kai Liu.
This research was primarily funded by the DOE Office of Science. The Advanced Light Source is a DOE Office of Science User Facility.
Additional Information
For more about the research of Charles Fadley, go here
For more about the research of Hendrik Bluhm, go here
For more about Berkeley Lab’s Advanced Light Source go here
For more about Berkeley Lab’s Chemical Sciences Division, go here
# # #
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit the Office of Science website at science.energy.gov/.
