Researchers from Berkeley Lab and Columbia University have created the world’s highest-performance single-molecule diode using a combination of gold electrodes and an ionic solution. (Image courtesy of Latha Venkataraman, Columbia University)
A team of researchers from Columbia University and Berkeley Lab’s Molecular Foundry has passed a major milestone in molecular electronics with the creation of a single-molecule diode that outperforms the best of its predecessors by a factor of 50.
“Using an ionic solution, two gold electrodes of dramatically different exposed surface areas, and a single symmetric molecule specially designed by the Luis Campos’ group at Columbia, we were able to create a diode that resulted in a rectification ratio, the ratio of forward to reverse current at fixed voltage, in excess of 200, a record for single-molecule devices,” says Latha Venkataraman, Associate Professor of Applied Physics at Columbia University.
“The asymmetry necessary for diode behavior originates with the different exposed electrode areas and the ionic solution,” says Jeff Neaton, Director of the Molecular Foundry, a U.S. Department of Energy (DOE) Office of Science User Facility. “This leads to different electrostatic environments surrounding the two electrodes and superlative single-molecule device behavior.”
Venkataraman, Campos and Neaton are the corresponding authors of a paper describing this research in Nature Nanotechnology. The paper is titled “Single-molecule diodes with high rectification ratios through environmental control.” The lead author is Brian Capozzi, a member of Venkataraman’s group who discovered the environmental asymmetric technique. Other co-authors are Jianlong Xia, Olgun Adak, Emma Dell, Zhen-Fei Liu and Jeffrey Taylor
With “smaller and faster” as the driving mantra of the electronics industry, single-molecule devices represent the ultimate limit in electronic miniaturization. In 1974, molecular electronics pioneers Mark Ratner and Arieh Aviram theorized that an asymmetric molecule could act as a rectifier, a one-way conductor of electric current. Since then, development of functional single-molecule electronic devices has been a major pursuit with diodes – one of the most widely used electronic components – being at the top of the list.
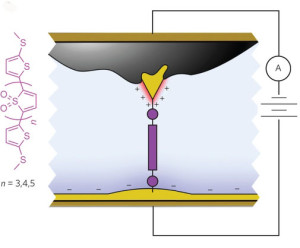
Schematic of the molecular junction created using asymmetric area electrodes which functions as a diode, allowing current to flow in one direction only.
A typical diode consists of a silicon p-n junction between a pair of electrodes (anode and cathode) that serves as the “valve” of an electrical circuit, directing the flow of current by allowing it to pass through in only one “forward” direction. The asymmetry of a p-n junction presents the electrons with an “on/off” transport environment. Scientists have previously fashioned single-molecule diodes either through the chemical synthesis of special asymmetric molecules that are analogous to a p-n junction; or through the use of symmetric molecules with different metals as the two electrodes. However, the resulting asymmetric junctions yielded low rectification ratios, and low forward current. The Columbia University groups, working together with Neaton and his group, have discovered a way to address both deficiencies.
Venkataraman and Campos and their respective research groups fabricated a high-performing rectifier from junctions made of symmetric oxidized thiophene derivatives with molecular resonance in nearly perfect alignment with the Fermi electron energy levels of the gold electrodes. Symmetry was broken by a substantial difference in the size of the area on each gold electrode that was exposed to the ionic solution. Owing to the asymmetric electrode area, the ionic solution, and the junction energy level alignment, a positive voltage increases current substantially; a negative voltage suppresses it equally significantly.
“Electron flow at molecular length-scales is dominated by quantum tunneling,” Neaton explains. “The efficiency of the tunneling process depends intimately on the degree of alignment of the molecule’s discrete energy levels with the electrode’s continuous spectrum. In a molecular rectifier, this alignment is enhanced for positive voltage, leading to an increase in tunneling, and is reduced for negative voltage. At the Molecular Foundry we developed an approach to accurately compute energy-level alignment and tunneling probability in single-molecule junctions to evaluate the hypothesis by the Columbia team. This method allowed myself and Zhenfei Liu to understand the diode behavior.”

Jeff Neaton directs the Molecular Foundry, a DOE Office of Science User Facility, and holds appointments with Berkeley Lab, UC Berkeley and Kavli ENSI.
“The ionic solution, combined with the asymmetry in electrode areas, allows us to control the junction’s electrostatic environment simply by changing the bias polarity,” Neaton says. “In addition to breaking symmetry, double layers formed by ionic solution also generate dipole differences at the two electrodes, which is the underlying reason behind the asymmetric shift of molecular resonance. The Columbia group’s experiments showed that with the same molecule and electrode setup, a non-ionic solution yields no rectification at all.”
The Columbia University and Berkeley Lab team believes their new approach to a single-molecule diode provides a general route for tuning nonlinear nanoscale-device phenomena that could be applied to systems beyond single-molecule junctions and two-terminal devices.
“We expect the understanding gained from this work to be applicable to ionic liquid gating in other contexts, and mechanisms to be generalized to devices fabricated from two-dimensional materials,” says Capozzi. “Beyond devices, these tiny molecular circuits are petri dishes for revealing and designing new routes to charge and energy flow at the nanoscale. What is exciting to me about this field is its multidisciplinary nature – the need for both physics and chemistry – and the strong beneficial coupling between experiment and theory.
Adds Neaton, “With the increasing level of experimental control at the single-molecule level, and improvements in theoretical understanding and computational speed and accuracy, we’re just at the tip of the iceberg with what we can understand and control at these small length scales.”
This research was primarily supported National Science Foundation. The computational work was supported by the DOE Office of Science and performed at National Energy Research Scientific Computing Center (NERSC), a DOE Office of Science User Facility also hosted at Berkeley Lab.
Additional Information
For more about the research of Latha Venkataraman go here
For more about the research of Luis Campos go here
For more about the research of Jeff Neaton go here
A news release from Columbia University that first reported this research can be viewed here
# # #
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit the Office of Science website at science.energy.gov/.