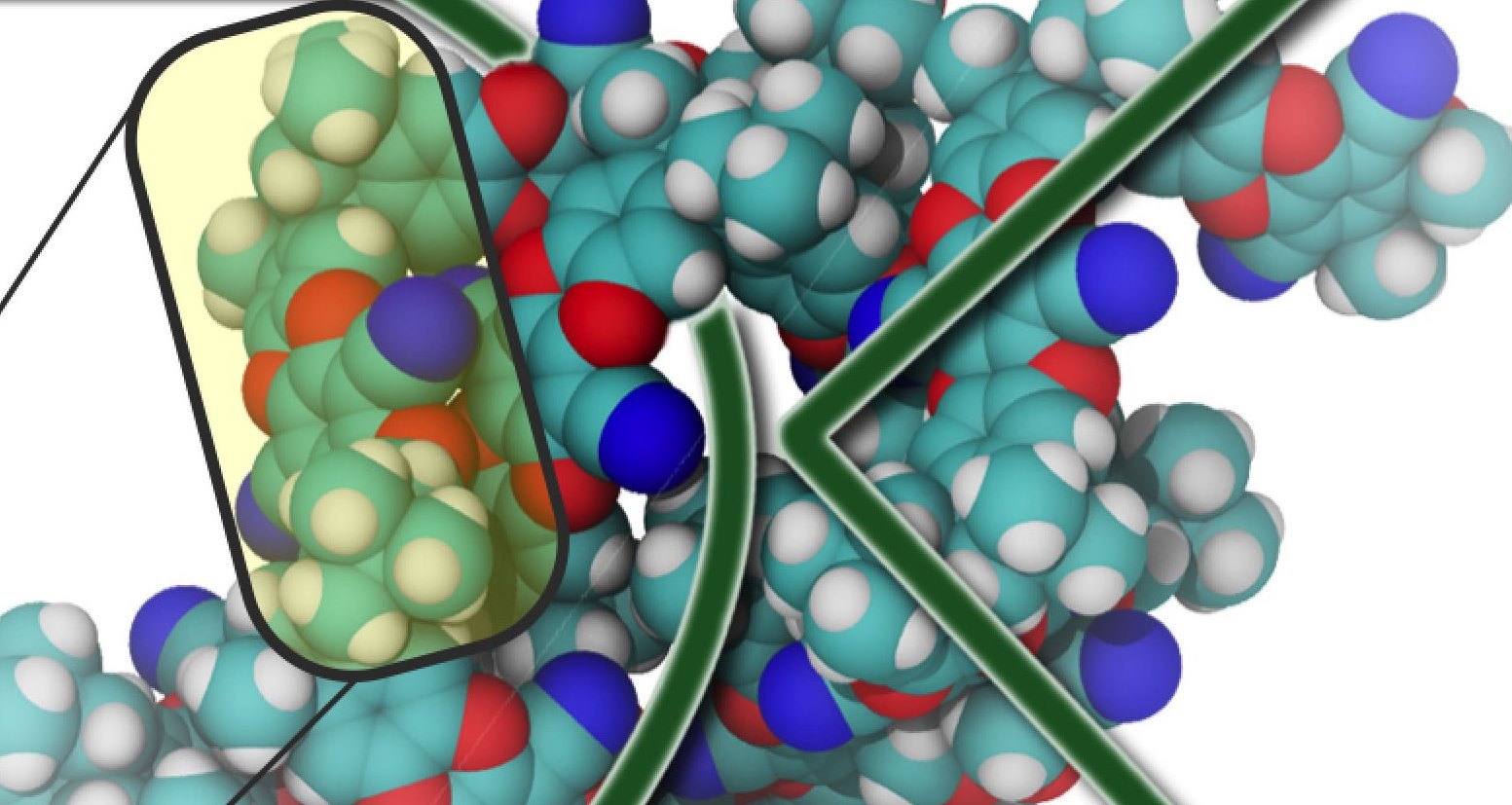
Membranes based on PIMs feature subnanometer-sized pores that allow smaller ions such as LiTFSI to pass through while blocking larger polysulfide ions (Li2Sx). This selectivity prevents unwanted crossovers that reduce battery lifetimes and performance.
Renewable sources of energy including solar and wind are fast gaining ground on fossil fuels, in part because of their sustainability and environmental benefits. A major issue, however, has been finding efficient ways to store the energy that renewables generate for use when the demand for energy is high. Lithium-sulfur batteries, which store electrical energy by transferring electrons to or from a sulfur electrode are well poised to provide high-density, long-term and low-cost electrochemical energy storage. The potential of lithium-sulfur batteries, has yet to be fully realized, however, due to the uncontrolled migration of soluble sulfur species through the membrane that separates the electrodes. This crossover of polysulfides reduces battery efficiency and lifetime.
Berkeley Lab researchers have found a solution to the polysulfide crossover problem with the development of a membrane made from polymers of intrinsic microporosity (PIMs). PIMs feature pore sizes of less than one nanometer in diameter, compared to the 17 nanometer pore size of typical membrane separators. This substantially smaller pore size provides highly selective control over the ions transported through the membrane. Smaller ions, like lithium and sodium are allowed to pass through the membrane while larger polysulfides are blocked. When integrated into lithium-sulfur cells, PIM membranes proved 500 times more effective at blocking the unwanted crossover of polysulfide ions than conventional membranes.
“In a proof-of-concept demonstration, we showed that our first-generation PIM membrane exhibited unprecedented blocking characteristics that dramatically reduced soluble polysulfide crossover and shuttling at the anode in lithium−sulfur batteries, even when the sulfur cathodes were prepared as flowable energy-dense fluids,” says Brett Helms, a Berkeley Lab staff scientist with the Molecular Foundry and principal investigator with the Joint Center for Energy Storage Research (JCESR), who led this research. “The blocking ability of our PIM membranes led to significantly longer-lasting batteries and other performance improvements.”

Brett Helms leads a team at the Molecular Foundry that is advancing a new platform for ion-selective membranes in batteries based on PIMs – polymers of intrinsic microporosity.
Helms is the corresponding author of a paper describing this research that’s been published in the journal Nano Letters. The paper is titled “Polysulfide-Blocking Microporous Polymer Membrane Tailored for Hybrid Li-Sulfur Flow Batteries.” Co-authors are Changyi Li, Ashleigh Ward, Sean Doris, Tod Pascal and David Prendergast.
“Guided by theoretical calculations, we developed and applied PIMs as a membrane platform for achieving high-flux, ion-selective transport in non-aqueous electrolytes,” Helms says. “The PIMs are synthesized in a single step and easily cast into large-area sheets with well-controlled pore structure and pore chemistry.”
Key to the micropore architecture of PIMs are two unique molecular features: the chemical bonds along their backbone are non-rotational; and rigid sharp bends are incorporated at regular intervals into at least one of the constituent monomers along their polymer chain. As a result of these two features, PIMs are amorphous but still exhibit high intrinsic microporosity and high surface area.
“Given that the pore size, pore chemistry and overall porosity for PIM membranes are tunable using molecular engineering and polymer processing, the membrane’s transport characteristics can be tailored to suit a broad spectrum of electrochemical devices, from batteries to fuel cells,” Helms says. “Our successful proof-of-concept demonstration suggests that a revolution in ion-transporting membranes is within reach.”
This research was funded by the DOE Office of Science. The computational portion of this work used the National Energy Research Scientific Computing Center (NERSC).
Additional Information
For more about the research of Brett Helms and his group go here