Technologies enabling energy-saving cool roofs, long-lived lithium-sulfur batteries, better biological data from X-ray experiments, safer drinking water, and reduced carbon dioxide in the atmosphere—developed by researchers at Berkeley Lab—have received annual awards presented by a scientific and engineering magazine.
R&D Magazine‘s R&D 100 Awards, established 54 years ago, recognize 100 technologies and services introduced in the previous year deemed most significant by an independent panel of judges. This year’s winners received the awards at a Nov. 3 event in Washington, D.C. The full list of winners is here: http://www.rd100conference.com/awards/winners-finalists/year/2016/.
A brief description of Berkeley Lab’s award-winning technologies follows:
Carbon Capture Simulation Initiative (CCSI) Toolset

This diagram describes the CCSI Toolset’s capabilities across the technology development cycle, from identifying the best carbon-capture concepts and determining which experiments to conduct, to scaling up the technology to pilot and demonstration, and then full-scale commercial deployment. (Credit: Berkeley Lab)
The Carbon Capture Simulation Initiative (CCSI), a partnership of national labs, industry and academic institutions, received an award for its computational tools and models that accelerate development of carbon-capture technologies by reducing cost, risk, and uncertainty. Carbon-capture technologies seek to prevent carbon dioxide generated by various sources from reaching the atmosphere, reducing its contribution to climate change.
This CCSI toolset includes rapid computational screening of promising carbon-capture concepts down to molecular scales; accelerated design and evaluation to reduce the time needed to design and troubleshoot new carbon-capture devices and processes; and risk management support that is based on comprehensive simulations and helps to gauge the impact of uncertainties.
The toolset is also intended to be responsive to experiment data and to identify where data gaps exist, which can serve as a guide on how to supplement existing models with new data.
Deb Agarwal of Berkeley Lab’s Computing Sciences Division led the effort. Researchers at the National Energy Technology Laboratory; Lawrence Livermore, Los Alamos, and Pacific Northwest national labs; Carnegie Mellon, West Virginia, Princeton, and Boston universities, and the University of Texas at Austin also partner in the research.
Compact Dynamic Beamstop (CDBS)

The Compact Dynamic Beamstop (CDBS) device, at left, designed to provide real-time information to improve X-ray crystallography experiments, is photographed here with a ballpoint pen tip at right. (Credit: Berkeley Lab)
Extracting 3-D structural information at the atomic scale from tiny, crystallized proteins is a critical tool in developing new therapies to treat cancers and fight life-threatening diseases such as HIV, Ebola, and tuberculosis. A Berkeley Lab team has built an important tool that can aid this protein research by providing important diagnostic information about the intense X-rays used to extract information from these delicate crystal samples.
The Compact Dynamic Beamstop, or CDBS, allows researchers to carefully monitor and optimize the X-ray dose for individual crystal samples by providing real-time information about the X-ray source. Obtaining a sufficient supply of protein crystals is a challenging, painstaking process, and CDBS can help to conserve the number of crystals required for experiments by helping researchers understand the X-ray beam properties in greater detail.
CDBS uses an array of precision-cut crystals, tightly packed into a 1-millimeter-diameter sleeve, that converts the X-ray beam into visible light. This visible light is delivered to sensors and provides important information that is used to capture the damaging portion of the X-ray beam while constantly monitoring the beam’s intensity. The device, several orders of magnitude smaller than alternative devices, also allows X-rays passing through the crystal to reach detectors. Diane Bryant and Simon Morton of Berkeley Lab’s Molecular Biophysics and Integrated Bioimaging (MBIB) Division developed the technology at the Berkeley Center for Structural Biology at the Advanced Light Source (ALS), a light source known as a synchrotron. The technology, which was licensed by MiTeGen LLC earlier this year, has been commercialized as the Sentinel Beamstop for use in X-ray beamlines at other synchrotrons around the world.
Cool Roof Time Machine

Berkeley Lab research associate Sharon Chen sprays roofing material with soiling mixture in a lab method that simulates the aging of roofing materials. (Photo by Roy Kaltschmidt/Berkeley Lab)
A Berkeley Lab-led team of scientists established a method to simulate soiling and weathering of roofing material, reproducing in the lab in only a few days what would naturally take three years. This “cool roof time machine” protocol has been approved by ASTM International, a widely referenced standards body, as a standard practice for the industry, and is expected to accelerate deployment of cool roofs, which have been shown to reduce a building’s energy use and mitigate the urban heat island effect.
The laboratory practice involves putting a piece of the roof material in a commercial weathering apparatus, which exposes the material to cycles of heat, moisture, and ultraviolet light, for one day. This “conditions” the material before soiling. Then a soiling apparatus developed at Berkeley Lab sprays a calibrated aqueous soiling mixture of dust, soot, particulate organic matter, and salts for about 10 seconds. After it dries, it goes back in the weathering apparatus for one more day, to simulate the cleaning effects of dew and rain.
The lead developers on the team were Ronnen Levinson, Hugo Destaillats, and Mohamad Sleiman of Berkeley Lab’s Energy Technologies Area, and Hashem Akbari of Concordia University, who also contributed to the work. (See here for a more complete list.)
Polymers of Intrinsic Microporosity (PIM) Membrane for Long-Lived Lithium-Sulfur (Li-S) Batteries
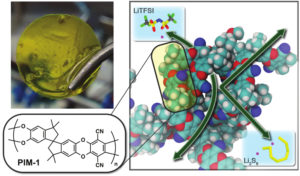
Berkeley Lab’s nano-sieving membranes are based on a new family of highly permeable microporous polymers. In lithium-sulfur batteries, these membranes can block soluble polysulfides from reaching the lithium anode, which provides for a longer battery-cycle life. (Credit: Berkeley Lab)
Next-generation lithium-sulfur (Li-S) batteries can potentially reduce costs as well as fire and environmental risks, compared to other battery technologies, and Berkeley Lab researchers have developed polymer membranes that enable extended battery-cycle life. The technology, which uses a type of polymer known as a PIM (polymer of intrinsic microscopy), selectively transports lithium ions through tiny pores while blocking larger polysulfide ions. This type of membrane can cost one to two orders of magnitude less than ceramic and other types of membranes per unit area, and also permit higher power.
Researchers report energy densities in its devices two times higher than a competing industry-leading technology, and this metric is important for reducing costs in grid-level energy storage devices. Also, the research team notes that sulfur is an environmentally preferred alternative to toxic heavy metals that are used in some batteries.
Brett Helms of Berkeley Lab’s Molecular Foundry led the development effort, which also involved research at the Joint Center for Energy Storage Research. Work on this PIM led to the creation of a company called Sepion, now a part of a technology entrepreneurship program at Berkeley Lab called Cyclotron Road.
Sustainable, Affordable Fluoride Removal (SAFR)
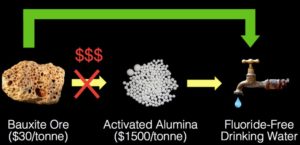
Refining bauxite to make activated alumina, a common fluoride adsorbent, is typically costly, energy intensive, and polluting. Now, a fluoride-removing process developed at Berkeley Lab has the potential to be locally sourced, easy to operate and maintain, and 50 times cheaper than existing treatment technologies. Click the image to enlarge. (Credit: Berkeley Lab)
Globally, some 200 million people are at risk of developing irreversible crippling deformities (such as dental/skeletal fluorosis) and other detrimental health effects by drinking groundwater contaminated with toxic levels of naturally occurring fluoride. Although many fluoride-removal technologies have been proven effective in the laboratory, few are sustainable in rural remote areas for various reasons, including cost and reliability.
Ashok Gadgil, of Berkeley Lab’s Energy Technologies Area, developed Sustainable and Affordable Fluoride Removal (SAFR), which uses mildly processed bauxite, an aluminum-rich ore available ubiquitously worldwide, as an adsorbent for remediating field-relevant fluoride concentrations in groundwater to reach the 1.5 milligrams-per-liter limit established by the World Health Organization as safe for drinking.
Because SAFR eliminates the costly and wasteful processing involved in refining bauxite into higher-end products for fluoride removal, it is a more sustainable method that also has lower energy costs and lower carbon emissions. This work involved research at Berkeley Lab’s Molecular Foundry.
Berkeley Lab’s ALS and Molecular Foundry are DOE Office of Science User Facilities.
View past Berkeley Lab winners of the R&D 100 Awards:
—Julie Chao contributed to this article.
