Arun Devaraj, a staff scientist at Pacific Northwest National Laboratory, loads a sample into a chamber in preparation for an atom probe tomography experiment. This method, which can provide a nanoscale 3-D map of a chemical element’s distribution in a material, was used in combination with other techniques, including X-ray experiments at Berkeley Lab, to study carbon-based deposits on a type of catalyst known as a zeolite. (Credit: PNNL)
Researchers have revealed new atomic-scale details about pesky deposits that can stop or slow chemical reactions vital to fuel production and other processes. This disruption to reactions is known as deactivation or poisoning.
The research team employed a combination of measurements, including X-ray experiments at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab), to gather the most detailed information yet on problematic carbon-based deposits called “coke,” and to find ways to prevent its formation or reduce its effects.
Detailed in the Nov. 23 edition of Scientific Reports, the study focused on deposits on HZSM-5, a common type of catalyst known as a zeolite that is used in biofuel production and in the refinery industry. Catalysts are materials that help to foster and speed up reactions without being used up in the process, and coke deposits in zeolites are a costly problem in petroleum refinement and in petrochemical production.
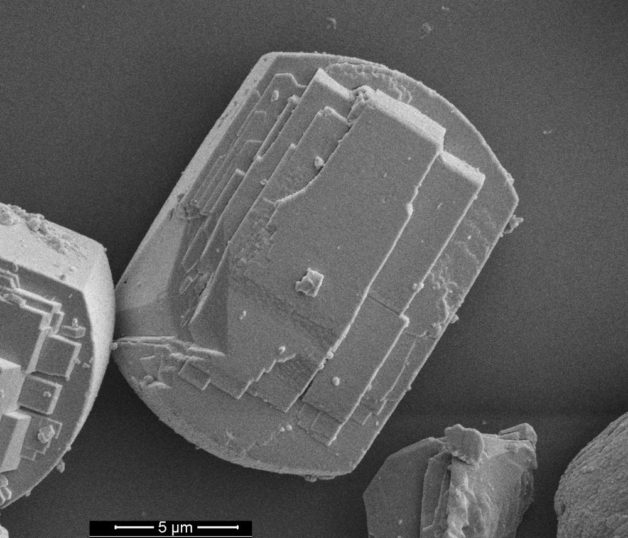
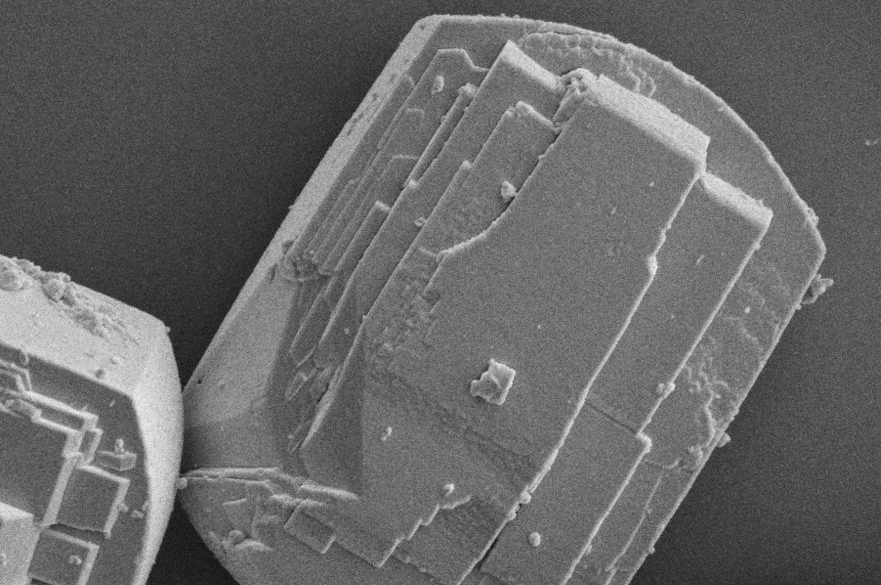
A scanning electron microscopy (SEM) image showing a type of catalyst called a zeolite that is used to convert ethanol to high-value fuels. The particle at center measures about 15 microns (millionths of a meter) in length. (Credit: PNNL)
This study was part of a larger R&D effort to convert ethanol and other compounds derived from biomass (organic matter) into renewable fuels.
A common way to restore the catalyst’s function is to heat and burn away the coke compounds, though this can cause permanent damage to the catalyst’s structure.
The latest study provides new insight about coke formation, both inside and on the surface of the catalyst, that could aid researchers in designing new catalysts or otherwise changing the chemical components and parameters to improve catalyst performance.
The multiple methods that the research team used to explore the chemistry of the coke deposits are helping to set the stage for a new suite of tools in the works at Berkeley Lab’s Advanced Light Source, dubbed AMBER (Advanced Materials Beamline for Energy Research), that will combine multiple X-ray and other experimental techniques in one place.
“The concept of AMBER is to have a variety of techniques available on the same beamline so you can tackle a problem more comprehensively—not only with X-rays, but also with techniques available at standard labs,” said Zahid Hussain, division deputy for scientific support at the ALS.
“You will be able to make materials, characterize them, and analyze them in one place. It is ‘one-stop shopping,'” Hussain said, adding that scientists will be able to study active chemistry with AMBER, too.
Pacific Northwest National Laboratory (PNNL) has partnered with Berkeley Lab to develop AMBER, and the latest study was conducted by PNNL researchers and provides an example of work that would benefit from this “multimodal” approach planned at AMBER, Hussain said. AMBER is expected to be ready for experiments within two years.
“We focused on where the coke is on an atomic scale, with high resolution,” said Arun Devaraj, a staff scientist at PNNL who was the study’s lead author. “We wanted to understand this coking mechanism and where it blocks the reaction and how it blocks it. By using a combination of techniques, we could compare them to one another and form a complete story.”

In the larger photo, Karthikeyan K. Ramasamy (at left), and Arun Devaraj, staff scientists at PNNL, prepare a flow reactor where ethanol is converted to high-value fuels using a type of catalyst known as a zeolite. In the smaller photo, Ramasamy holds three vials containing ethanol (left), zeolite pellets (middle), and high-value fuel (right). (Credit: PNNL)
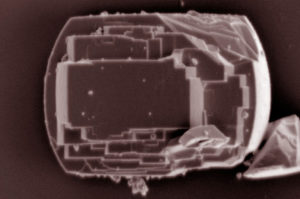
An X-ray technique called X-ray absorption spectroscopy, performed at the ALS, along with a form of nuclear magnetic resonance spectroscopy at PNNL, provided a chemical signature for the coke molecules. Also, the research team used a method called atom probe tomography (APT) at PNNL to collect 3-D reconstructed images showing how carbon-containing molecules responsible for coking were distributed in the pores of the catalyst.
Researchers found that an uneven distribution of aluminum in the fresh zeolite catalyst caused an uneven distribution of coke deposits during ethanol-conversion reactions. The coke buildup appears to be associated with aluminum-rich regions within the pores of HZSM-5.
Karthikeyan K. Ramasamy, also a PNNL staff scientist, said this information about the coke formations and locations will be useful as the team explores other ways to convert ethanol, a common additive in gasoline, and other compounds generated from renewable resources into fuel sources compatible with existing fueling systems.
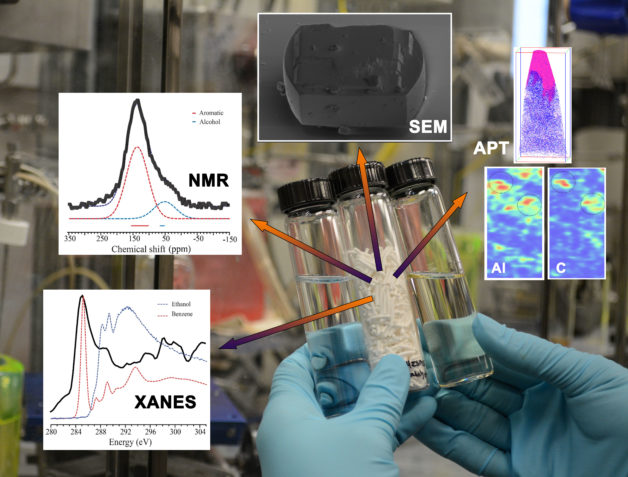
Scientists used a combination of techniques to study carbon-based deposits called coke, which can disrupt chemical reaction, on a type of catalyst called a zeolite that is shown in pellet form in the middle vial. The pellets are used in a chemical process that converts ethanol (vial on the left) into high-value fuels (vial on the right). The tools used to explore the coke deposits on the catalyst included: atom probe tomography (APT), a type of X-ray adsorption spectroscopy (XANES), a type of nuclear magnetic resonance (NMR), and scanning electron microscopy (SEM). (Credit: PNNL)
He also said a team of researchers at PNNL is working on several pathways to convert biomass into higher-value compounds. “Understanding coke molecules in zeolites will provide broad benefits across the refinery and renewable energy industries, and zeolite is one of the most highly utilized catalysts,” he said.
Hussain said that in addition to chemistry research, the AMBER station now in development at Berkeley Lab’s ALS will be used for battery and artificial photosynthesis work, and to support other renewable energy research.
Some of the work was performed at PNNL’s Environmental Molecular Sciences Laboratory (EMSL). The Advanced Light Source and EMSL are DOE Office of Science User Facilities.
The work was supported by the Bioenergy Technologies Office within DOE’s Office of Energy Efficiency & Renewable Energy and by PNNL’s Laboratory Directed Research and Development Program.
# # #
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.