There’s a known rule-breaker among materials, and a new discovery by an international team of scientists adds more evidence to back up the metal’s nonconformist reputation. According to a new study led by scientists at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and at the University of California, Berkeley, electrons in vanadium dioxide can conduct electricity without conducting heat.
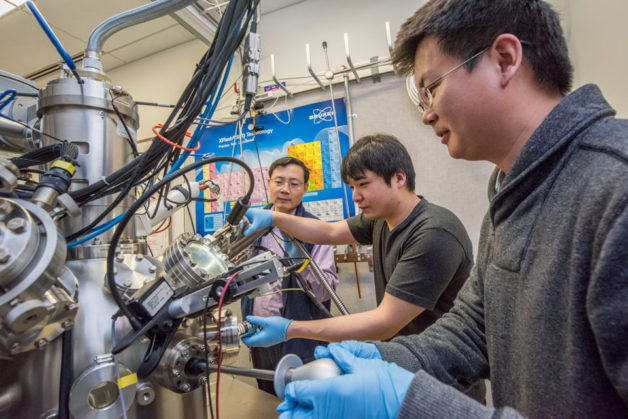
Berkeley Lab scientists Junqiao Wu, Changhyun Ko, and Fan Yang (l-r) are working at the nano-Auger electron spectroscopy instrument at the Molecular Foundry, a DOE Office of Science User Facility. They used the instrument to determine the amount of tungsten in the tungsten-vanadium dioxide (WVO2) nanobeams. (Credit: Marilyn Chung/Berkeley Lab)
The findings, to be published in the Jan. 27 issue of the journal Science, could lead to a wide range of applications, such as thermoelectric systems that convert waste heat from engines and appliances into electricity.
For most metals, the relationship between electrical and thermal conductivity is governed by the Wiedemann-Franz Law. Simply put, the law states that good conductors of electricity are also good conductors of heat. That is not the case for metallic vanadium dioxide, a material already noted for its unusual ability to switch from an insulator to a metal when it reaches a balmy 67 degrees Celsius, or 152 degrees Fahrenheit.
“This was a totally unexpected finding,” said study principal investigator Junqiao Wu, a physicist at Berkeley Lab’s Materials Sciences Division and a UC Berkeley professor of materials science and engineering. “It shows a drastic breakdown of a textbook law that has been known to be robust for conventional conductors. This discovery is of fundamental importance for understanding the basic electronic behavior of novel conductors.”
In the course of studying vanadium dioxide’s properties, Wu and his research team partnered with Olivier Delaire at DOE’s Oak Ridge National Laboratory and an associate professor at Duke University. Using results from simulations and X-ray scattering experiments, the researchers were able to tease out the proportion of thermal conductivity attributable to the vibration of the material’s crystal lattice, called phonons, and to the movement of electrons.
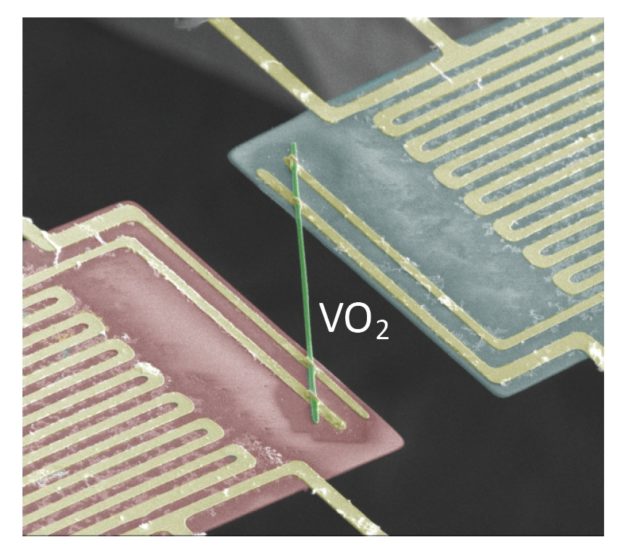
Vanadium dioxide (VO2) nanobeams synthesized by Berkeley researchers show exotic electrical and thermal properties. In this false-color scanning electron microscopy image, thermal conductivity was measured by transporting heat from the suspended heat source pad (red) to the sensing pad (blue). The pads are bridged by a VO2 nanobeam. (Credit: Junqiao Wu/Berkeley Lab)
To their surprise, they found that the thermal conductivity attributed to the electrons is ten times smaller than what would be expected from the Wiedemann-Franz Law.
“The electrons were moving in unison with each other, much like a fluid, instead of as individual particles like in normal metals,” said Wu. “For electrons, heat is a random motion. Normal metals transport heat efficiently because there are so many different possible microscopic configurations that the individual electrons can jump between. In contrast, the coordinated, marching-band-like motion of electrons in vanadium dioxide is detrimental to heat transfer as there are fewer configurations available for the electrons to hop randomly between.”
Notably, the amount of electricity and heat that vanadium dioxide can conduct is tunable by mixing it with other materials. When the researchers doped single crystal vanadium dioxide samples with the metal tungsten, they lowered the phase transition temperature at which vanadium dioxide becomes metallic. At the same time, the electrons in the metallic phase became better heat conductors. This enabled the researchers to control the amount of heat that vanadium dioxide can dissipate by switching its phase from insulator to metal and vice versa, at tunable temperatures.
Such materials can be used to help scavenge or dissipate the heat in engines, or be developed into a window coating that improves the efficient use of energy in buildings, the researchers said.
“This material could be used to help stabilize temperature,” said study co-lead author Fan Yang, a postdoctoral researcher at Berkeley Lab’s Molecular Foundry, a DOE Office of Science User Facility where some of the research was done. “By tuning its thermal conductivity, the material can efficiently and automatically dissipate heat in the hot summer because it will have high thermal conductivity, but prevent heat loss in the cold winter because of its low thermal conductivity at lower temperatures.”
Vanadium dioxide has the added benefit of being transparent below about 30 degrees Celsius (86 degrees Fahrenheit), and absorptive of infrared light above 60 degrees Celsius (140 degrees Fahrenheit).
Yang noted that there are more questions that need to be answered before vanadium dioxide can be commercialized, but said that this study highlights the potential of a material with “exotic electrical and thermal properties.”
While there are a handful of other materials besides vanadium dioxide that can conduct electricity better than heat, those occur at temperatures hundreds of degrees below zero, making it challenging to develop into real-world applications, the scientists said.
Other co-lead authors of the study include Sangwook Lee at Kyungpook National University in South Korea, Kedar Hippalgaonkar at the Institute of Materials Research and Engineering in Singapore, and Jiawang Hong at the Beijing Institute of Technology in China. Lee and Hippalgaonkar started work on this paper as postdoctoral researchers at UC Berkeley. Hong began his work as a postdoctoral researcher at Oak Ridge National Laboratory. The full list of authors is available online.
Additional support for this work came through the use of facilities supported by the Electronic Materials Program at DOE’s Office of Science.
###
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel Prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit http://www.lbl.gov.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
