Contact: Dan Krotz, (510) 486-4019, [email protected]
A compound synthesized for the first time by Berkeley Lab scientists could help to push nanotechnology out of the lab and into faster electronic devices, more powerful sensors, and other advanced technologies.
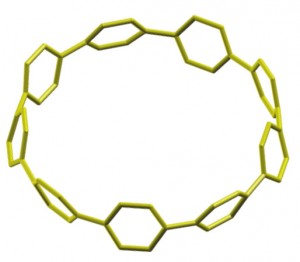
The scientists developed a hoop-shaped chain of benzene molecules that had eluded synthesis, despite numerous efforts, since it was theorized more than 70 years ago.
The much-anticipated debut of the compound, called cycloparaphenylene, couldn’t be better timed. It comes as scientists are working to improve the way carbon nanotubes are produced, and the newly synthesized nanohoop happens to be the shortest segment of a carbon nanotube. Scientists could use the segment to grow much longer carbon nanotubes in a controlled way, with each nanotube identical to the next.
“The holy grail in this field is to come up with a way to make a single type of carbon nanotube on demand,” says Ramesh Jasti, a postdoctoral researcher in Berkeley Lab’s Materials Sciences Division. “And this compound moves us toward this goal of rational synthesis.”
Jasti conducted the research at the Molecular Foundry, a U.S. Department of Energy User Facility located at Berkeley Lab that provides support to nanoscience researchers around the world. He worked with Carolyn Bertozzi, director of the Molecular Foundry, as well as other Berkeley Lab scientists.
To synthesize the elusive cycloparaphenylene, the team developed a relatively simple, low-temperature way to bend a string of benzene rings — which normally resist bending — into a hoop. The result is a structure that is as unusual as it is potentially useful. It should be flat, but it’s circular. And it’s poised to improve the way one of most promising stars in nanotechnology is produced.
Carbon nanotubes are hollow wires of pure carbon about 50,000 times narrower than a human hair. They can be semiconducting or metallic depending on how they’re structured. Their unique properties could usher in a new era of faster and smaller computers, or tiny sensors powerful enough to detect a single molecule.
But carbon nanotubes haven’t made inroads into the electronics industry and other sectors because they’re difficult to make in large quantities. They’re currently produced in batches, with only a handful of nanotubes in each batch possessing the desired characteristics. This shotgun approach works fine in the lab, but it’s too inefficient for commercial applications.
Cycloparaphenylene offers a more targeted approach. The family of compounds forms the smallest carbon hoop structure with a set diameter and set orientation of benzene molecules, which are the two variables that determine a nanotube’s electronic properties. Because of this, cycloparaphenylene molecules could be used as seeds or templates to grow large batches of carbon nanotubes with just the right specifications.
This combination of precision and high yield will be needed if carbon nanotubes are to make the jump from the lab to the commercial sector. In order for carbon nanotubes to replace silicon wafers in electronics, for example, they’ll need to be just as unblemished as silicon wafers, and just as easy to make in large numbers.
“This compound, which we synthesized for the first time, could help us create a batch of carbon nanotubes that is 99 percent of what we want, rather than fish out the one percent like we do today,” says Jasti. “The idea is to take the smallest fragment of a carbon nanotube, and use that to build tubular structures.”
The research, which is published in a recent issue of the Journal of the American Chemical Society, was funded in part by the Department of Energy.
Additional Information:
- Learn more about the Molecular Foundry.