Contact: Paul Preuss, (510) 486-6249, [email protected]
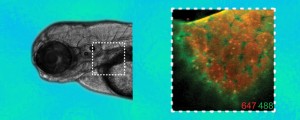
Two kinds of fluorescent probes “clicked” onto specially tagged glycans show changes in patterns of expression as a zebrafish embryo evolves. In this image of the pectoral fin region, the glycans on the cell surfaces 60 hours after fertilization are shown in orange, while “new” glycans expressed an hour later are shown in green.
“Imaging the glycome,” Carolyn Bertozzi’s Proceedings of the National Academy of Science inaugural article, written with colleague Scott T. Laughlin, appeared this January. It’s a fascinating review of one of the major themes of her scientific career, detailing the development of a series of techniques by which Bertozzi’s lab manipulates the ubiquitous biomolecules known as glycans, sugars that occur throughout living things and are particularly abundant on the surfaces of cells.
Cell-surface glycans are involved in binding bacteria and viruses to host cells as well as with the adhesion of white blood cells during an inflammatory response to infection. Inside the cell, glycans regulate processes including transcription and translation. And they perform many other functions. The glycome is the totality of the glycans a cell produces, so-called by analogy to the genome, the proteome, the transcriptome, and other “omes.”
It’s no surprise, then, that Bertozzi’s glycans modifications have diverse applications, including labeling cells for diagnostic purposes, or as targets for drugs. But one of their most intriguing uses is visualizing the molecular processes by which glycans play their myriad roles. Spectacular recent examples of imaging the glycome include visually tracking the evolving arrangements of glycans during the development of zebrafish.
Unlike proteins, glycans are not directly programmed by genes. They can’t be labeled using genetic methods, in the way that a protein can be made to fluoresce by genetically combining its gene with that of a fluorescent protein. Instead, Bertozzi has devised ways to rig target glycans with reactive groups called “chemical reporters.” Probes such as fluorescing molecules (fluorophores) are then sent in to react with the reporters.
In the beginning
Bertozzi is the director of the Molecular Foundry and a member of Berkeley Lab’s Materials Sciences and Physical Biosciences Divisions, as well as a professor of Chemistry and Molecular and Cell Biology at UC Berkeley, where she is a Howard Hughes Medical Institute investigator. Just over a decade ago, in what she called “an equal combination of cell biology and synthetic organic chemistry,” she and her colleagues devised the key component of cell-surface engineering, the use of natural biological processes to plant artificial markers on the surfaces of living cells.
In essence, Bertozzi’s group tricked a colony of living cells into ingesting synthetic sugars with novel chemical properties – they chose a synthethic sialic acid that expressed a ketone group, which is otherwise absent from cell surfaces. The cells then incorporated these modified sugars into the glycans they expressed on their surfaces. The results were cells that had decorated themselves with unusual reporters, which reacted with other chemical groups — in this case, hydrazide groups — for labelling the cells as drug targets, or for adhering them to nonbiological materials such as medical implants, or for other functions, including imaging.
But the ketone reporter had its disadvantages. Although absent from cell surfaces, ketones occur naturally within metabolites that are commonly found inside cells. For more precision, and to make it possible to engineer the cell’s interior as well as its surface, new tags were needed that would react exclusively with nonbiological groups.
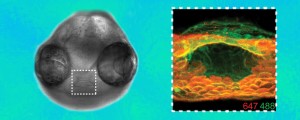
Azide groups are the key chemical reporters in the synthetic glycans linked to the fluorescent probes in the zebrafish images. Glycan patterns on cells in the mouth region are shown as they change beginning at 60 hours after fertilization (orange) and again an hour later (green).
For this purpose Bertozzi chose azides, which are essentially unreactive with any other biological molecules outside or inside the cell. Like ketones, the cell happily ingests and expresses sugars fitted with azide groups. The next step was to find a way to latch the reporter azides in the expressed sugars to synthetic probe molecules.
“We need reactions that proceed in a watery environment at body temperature, that are highly selective, and that won’t interfere with normal cell processes,” Bertozzi said. She calls these kinds of reactions bioorthogonal, meaning they are (figuratively) “at right angles” to biological processes. “That means we had to create new reactions.”
Bertozzi started with the classic Staudinger reaction, a highly specific reaction between an azide and a phosphine that forms a compound called an aza-ylide. The Staudinger reaction meets the requirements of orthogonality, but unfortunately the resulting aza-ylides fall apart in the cell’s watery environment almost as quickly as they form. Bertozzi’s skills as a synthetic organic chemist solved the problem with adjustments to the phosphine’s structure, ensuring that the cell’s azide-equipped glycans would bind stably to any synthetic molecule equipped with the newly designed phosphine group. She had transformed the Staudinger reaction into what she named, in Staudinger’s honor, the Staudinger ligation.
The Staudinger ligation proved particularly powerful for imaging cultured cells after Bertozzi and her colleagues developed “smart” probes, fluorophores that turn on only after they have reacted with the azide reporter in the target glycans. By reducing ambient background fluorescence, this and other modifications yield images with high sensitivity.
Life in the fast lane
The Staudinger ligation is safe for living things and has been used for tagging glycoproteins in mice. But compared to the speed at which most processes involving glycans proceed, the Staudinger ligation is much too slow.
Enter “click” chemistry, so-called by chemist Barry Sharpless of the Scripps Research Institute to describe a set of reactions in which small molecules rapidly assemble, snapping themselves together like Legos. The best known click-chemistry reaction is that between alkynes and by-now-familiar azides; it’s fast, reliable, and occurs in an aqueous environment at room temperature. The catch is, the original version has to be catalyzed by copper, which kills cells.
What Bertozzi needed was click chemistry without the copper. She and her group proceeded to redesign the azide-alkyne reaction using a ring-shaped molecule called a cyclooctyne (difluorinated cyclotoctyne, DIFO for short) in place of the usual terminal alkyne, whose triple bond comes at the end of a long line of atoms. In the new compound, the triple bond is embedded in DIFO’s eight-member, stop-sign-shaped ring.
“Triple bonds want to be linear, and when you put them in a ring you bend them,” Bertozzi explains. “And when you bend them, the strain makes them more reactive.” Consequently, DIFO reacts with azides 30 to 60 times faster than the Staudinger ligation.
The result is copper-free click chemistry, which can be used to probe glycans or any other biomolecule that can be labeled with an azide. Recently, Bertozzi and her colleagues, including Laughlin, used copper-free click chemistry for the first studies of glycan imaging in a living organism, the zebrafish.
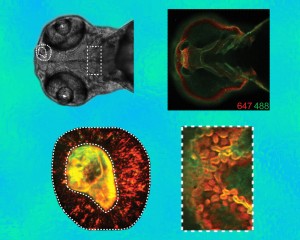
Two kinds of fluorescent probes linked with the copper-free click-chemistry reagent DIFO were used to label two distinct time points as patterns of glycan expression evolve – in cells throughout the organism (upper right), in the olfactory region (lower left), and in the region of the jaw (lower right). Orange is the pattern at 60 hours after fertilization and green the pattern an hour later.
“We start the zebrafish embryos in a culture dish, in the presence of these synthetic azido-sugars; they take up the sugars from the medium, which are part of the nutrient pool, and metabolize them – and the synthetic sugars end up sprinkled in and among the cell-surface glycans within the developing organs and tissues.” Bertozzi explains that because zebrafish embryos are transparent throughout most of their development, they are convenient models for imaging vertebrate development.
“Then we take those same zebrafish embryos, and at different time points we expose them to DIFO, the copper-free click chemistry reagent, linked to a fluorescent probe. The DIFO finds the azides in the azido-sugars, and we can see the sugars because of the fluorescence of the probe.” In this way Bertozzi’s group performed a fluorescence microscopy analysis of changes in the distribution and abundance of certain types of sugars as a function of time during zebrafish development.
“We found some interesting and totally unexpected patterns, as the sugars moved and clustered in certain areas.” Bertozzi says, “It’s like putting a new telescope up to the stars and seeing something new. And then you have to figure out what it is.”
Thus one result of Bertozzi’s methods for tagging and probing glycans is a totally new way of visualizing vertebrate development, opening unexpected vistas for further research. For engineering of cells both in vitro and in vivo, it’s just the beginning.
Additional information
“Imaging the glycome,” by Scott T. Laughlin and Carolyn R. Bertozzi, appears in the 6 January 2009 issue of Proccedings of the National Academy of Sciences and is available online to subscribers at http://www.pnas.org/content/106/1/12.abstract?sid=34f7217a-6e4f-45c3-9e9f-274c0357f09c
“In vivo imaging of membrane-associated glycans in developing zebrafish,” by Scott T. Laughlin, Jeremy M. Baskin, Sharon L. Amacher, and Carolyn R. Bertozzi1, appears in the 2 May 2008 issue of Science magazine and is available online to subscribers at http://www.sciencemag.org/cgi/reprint/320/5876/664.pdf
More on copper-free click chemistry is at http://www.lbl.gov/Science-Articles/Archive/MSD-click-chemistry.html
More on cell-surface engineering in living animals is at http://www.lbl.gov/Science-Articles/Archive/MSD-cell-surface-eng.html
More on the Staudinger ligation is at http://www.lbl.gov/Science-Articles/Archive/staudinger-ligation.html
More on the basics of using natural biological processes to plant artificial markers on the surfaces of living cells is at http://www.lbl.gov/Science-Articles/Archive/custom-cells.html