Contact: Lynn Yarris (510) 486-5375, [email protected]
The immediate lure of the hydrogen highway – vehicles powered by hydrogen in the form of batteries or fuel – is that the only exhaust product is water vapor. Even better, if the hydrogen is produced through solar water splitting – the process by which sunlight is used to extract hydrogen from liquid water – then hydrogen becomes not only a clean but also a renewable source of energy. Getting to the hydrogen highway is a high priority for both the federal and the California state governments. Berkeley Lab researchers with the Helios Solar Energy Research Center are finding that the best route forward could be via the nano road
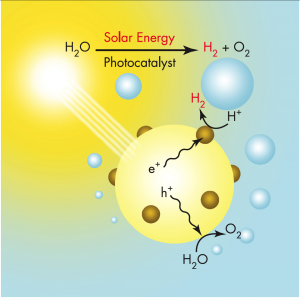
Solar water splitting is the process by which energy in solar photons is used to break down liquid water into molecules of hydrogen and oxygen gas. Hydrogen produced through solar water splitting is sustainable and does not emit carbon into the atmosphere.
Peidong Yang, a leading authority on nanoscience who holds joint appointments with Berkeley Lab and UC Berkeley, has been one of the principal scientific drivers behind the development of semiconductor nanowires, essentially one-dimensional strips of materials whose width measures only one-thousandth that of a human hair but whose length may stretch several microns. Because the movement of electrons through them is confined, nanowires display properties that can be quite different from those of bulk or three-dimensional crystals. Such unique properties pave the way for nanowires fashioned from combinations of silicon with other materials to play a potentially big role in the harvesting of solar energy for water splitting.
“Semiconductor nanowires and their heterojunctions, if properly designed, can have optimized light absorption, high charge mobility, efficient charge separation and collection, and reduced recombination (compared to bulk or other nanoparticle approaches, all of which are crucial in order to develop an efficient solar cell or an integrated system for solar water splitting,” Yang says.
Hydrogen is the most abundant element in the universe but because it combines with just about any other type of atom, pure hydrogen must be extracted from other substances. Most pure hydrogen today comes from natural gas or methane, a fossil fuel. This is expensive and adds carbon emissions to the atmosphere. Solar water splitting is the ideal way to produce pure hydrogen, however, achieving efficient photoelectrolysis is a major stumbling block as it requires a photovoltaic (PV) anode that can absorb light and generate an electrical current (charge separation) but won’t be corroded by water. Silicon, the most widely used PV material today is a proficient photon collector but readily corrodes in water. The ceramic titanium oxide has drawn a lot of scientific attention because of its resistance to water but suffers from its poor efficiency as a light harvester. Yang and members of his research group including Yun Jeong Hwang and Akram Boukai, have found a way to boost this efficiency through nanowires.
“The efficiency at which titanium oxide can convert solar energy to hydrogen is quite low because of its large bandgap,” Yang said, in a talk he gave at the spring 2009 national meeting of the American Chemical Society in Salt Lake City. “We prepared highly dense vertical arrays of nanowires consisting of a silicon core coated with a protective shell of titanium oxide. When these nanowire arrays were grown to heights of 20 microns, their photocurrent was more than doubled compared to planar wafers of silicon and titanium oxide.”
Highly dense vertical arrays of nanowires made from silicon and titanium oxide and measuring 20 microns in height show promise for the effiecient production of hydrogen through solar water splitting.
Yang attributed the superior performance of the 20 micron nanowire arrays to the fact that the arrays, like trees in a dense forest, suppress sunlight reflection. Also like trees in a dense forest, the arrays provide more surface area for photon absorption. Yang and his colleagues tested both n-type and p-type heterojunctions of the silicon core-titanium oxide shell nanowires and found that the n-type junctions generated the highest photocurrents (2.5 times that of the silicon titanium oxide planar wafers) and the lowest onset potential because of enhanced charge separation and minimized charge recombination.

Peidong Yang is an award winning chemist and leading authority on nanoscience who holds appointments with Berkeley Lab’s Molecular Foundry and Materials Sciences Division and with the UC Berkeley Chemistry Department.
“The n/n heterojunction is a promising structure for solar water splitting since the photovoltage at the junction can compensate for the lower energy level of the conduction band of the shell semiconductor,” Yang said. “Also, the n/n heterojunction can increase the potential efficiency of the photovoltaic cell due to a higher open circuit voltage and higher photocurrent.”
This research was funded by the Director, Office of Basic Energy Sciences, Chemical Sciences, Materials Sciences and Engineering Division, of the U.S. Department of Energy
Additional Information
For more about the research of Peidong Yang and his group, visit the Website at http://www.cchem.berkeley.edu/pdygrp/main.html
For more information about Helios SERC, visit the Website at http://www.lbl.gov/LBL-Programs/helios-serc/index.html