Contact: Paul Preuss, [email protected]
John Spence, a physicist at Arizona State University, is a longtime user of the Advanced Light Source at Lawrence Berkeley National Laboratory, where he has contributed to major advances in lensless imaging. It’s a particularly apt propensity for someone who works with x-rays, since they can’t be focused with ordinary lenses.
As new light sources evolve to produce brighter x-rays in faster pulses, lensless imaging becomes ever more critical for science. Among the promises of superbright, ultrafast x-ray pulses is the ability to solve the structure of the complicated molecules from which our bodies are made. All living things are made of proteins and nucleic acids, but relatively few of the atomic structures of the thousands, perhaps millions, of varieties of proteins are known.
The Linac Coherent Light Source (LCLS) will soon begin operation at the SLAC National Accelerator Laboratory in Palo Alto, California, using energetic electrons from a linear accelerator to produce coherent x-rays with an instrument called a free electron laser (FEL). The x-rays will be delivered 120 times a second in pulses only a tenth of a trillionth of a second long – about the time it takes light to travel the width of a human hair. These brief, bright pulses offer a novel approach to the problem of protein structure.
Unfolding the origami
Proteins begin as strings of amino acids that fold themselves into an amazing variety of origami-like structures, whose bumps and crannies and distribution of electrical charges determine how they act individually or fit together to form complex molecular machines. Simple organisms like viruses often consist of a few proteins fitted together to enclose a thread of DNA or RNA.
Proteins are usually large molecules containing many thousands of atoms. Drug molecules are much smaller, and do their work by attaching themselves to the larger protein molecules. A knowledge of the arrangement of a protein’s atoms is therefore a great help to drug designers, who like to understand how a drug molecule will dock with a protein to promote or inhibit its activity, or cripple the organism of which it is a part.
Until now, the best way to solve the structure of a protein or virus has been with x ray crystallography. The crystal consists of many copies of the protein or virus arranged in regular order. As the crystal rotates in the x-ray beam, x-rays scatter off the atoms and reveal – once these complex diffraction patterns have been converted into a 3-D image by computers – how the electrons, and thus the atoms, are arranged.
But many proteins can’t be crystallized at all, and others are so difficult to crystallize it’s virtually impossible to obtain crystals large enough to use in today’s light sources.
Ultrafast, ultrabright x-rays offer a way past this dilemma. The idea is that a quick pulse of tightly focused x-rays can be diffracted from a microcrystal or even a single protein or virus in solution. The pulse is so brief that it comes and goes before any of the atoms can move, freezing their orientation like a strobe light. Just as important, a sufficiently brief pulse may terminate before radiation damage effects can start. In this way it can outrun radiation damage, always one of the fundamental limitations to imaging in biology.
Another quick pulse could be diffracted from another copy of the protein in a different orientation. As the process is repeated, diffractions from different angles give the overlapping views needed for the computer to construct a 3-D image of the structure.
It’s a great idea, but as Spence notes, there are a few problems. “So as not to scatter, the x-ray beam has to be in a high vacuum, but a protein or virus in its natural state is usually wet. As in T. S. Eliot’s Wasteland, water is life. How do we maintain the protein or virus in an aqueous environment inside the vacuum?”
Shot from a microcannon
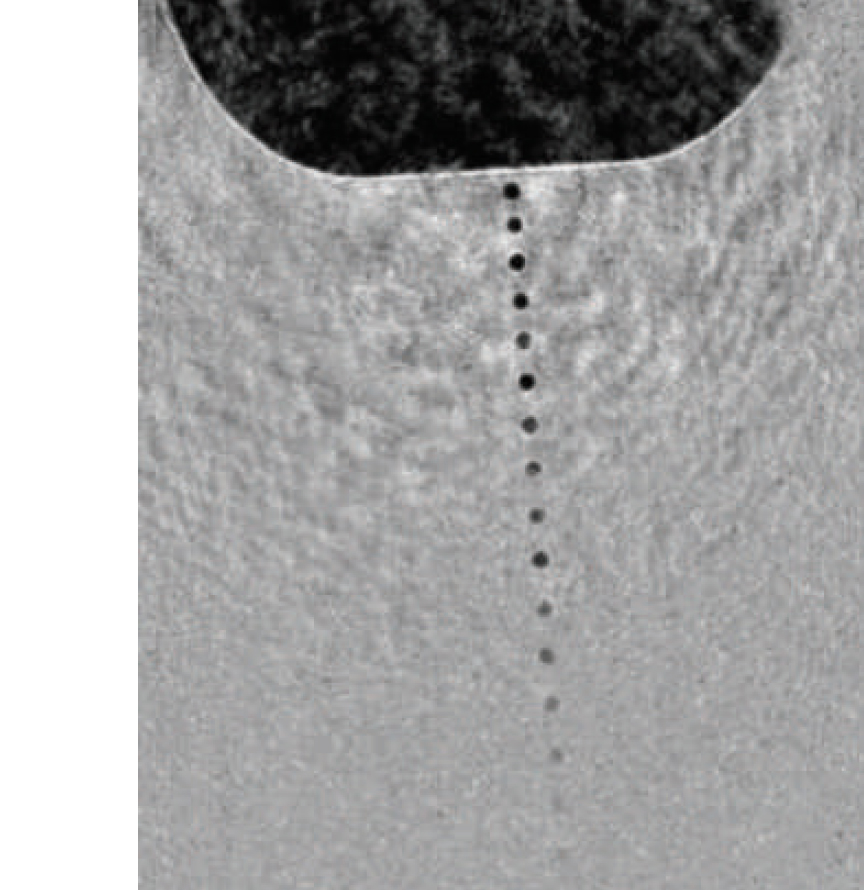
The answer was what Spence calls a “particle gun, like an ink-jet printer,” designed to inject a beam of water droplets across the tightly focused x-ray beam in single file, each droplet so small it contains only a single protein or virus. He and colleagues Bruce Doak and Uwe Weierstall of ASU designed a nozzle that can fire liquid droplets, each less than a millionth of a meter in diameter (one micrometer), faster than hundreds of thousands of times a second. The sample jet is designed to shoot droplets right through a pulsed beam of x-rays a billion times brighter than any ever created in a light source before.
It wasn’t easy. Nozzles made of solid material like glass invariably clog up, limiting droplets to at best 20 micrometers across. What Spence and his colleagues wanted was a jet of particles less than a micrometer in size. ASU postdoc Dan DePonte has done most of the recent hard work needed to make it all function.

The frequency at which droplets emerge is controlled by an acoustic trigger, which can be tuned so that each droplet containing a protein or virus meets an incoming pulse of x-rays.
Back in 1878 Lord Rayleigh, a professor of experimental physics at Cambridge University, discovered that a smooth, cylindrical jet of liquid emerging from an orifice spontaneously breaks up to form a train of spherical droplets. In the late 1990s, physicist Alfonso Gañán-Calvo of the University of Seville found a way to surround the streaming liquid with pressurized gas to make a co-flowing liquid sheath. By adjusting gas and liquid pressure and other parameters, he was able to create a “virtual nozzle” that could shrink the diameter of the liquid jet to a thread so small it would not clog the physical aperture of the tube. In effect, the gas sheath acts to focus the liquid stream.
Spence and his colleagues needed a true microthread of liquid, however, one that produced droplets sized a millionth of a meter or less. In their nozzle, liquid flows through a narrow capillary inside the tube through which the gas flows; the liquid issues from the capillary some distance from the opening in the outer tube, so the gas surrounds it, then increases speed and pressure as it approaches the opening, squeezing and accelerating the thin stream of liquid until it is so small that the proteins or viruses dissolved in the liquid can only fit into the droplets one at a time.
And the nozzle won’t clog, because even a particle bigger than the sample protein or virus – bigger than the stream of liquid itself – can still fly through the glass nozzle without hitting the walls and getting stuck.
The frequency at which the droplets emerge can be controlled by an oscillator the researchers call an “acoustic trigger.” Tuning the acoustic trigger adjusts the frequency so that each droplet containing a protein or virus meets an incoming pulse of x-rays.
The entire device – which the researchers call a gas dynamic virtual nozzle (GDVN) – is only about a millimeter in diameter (not counting feed lines and cables) and fits to the side of the beamline’s vacuum chamber. After passing through the beam, the liquid droplets and the gas (typically carbon dioxide) freeze in a trap opposite the injection point, without significantly reducing the vacuum.

The liquid flows through a capillary, issuing some distance from the opening in the outer tube through which the gas flows. Approaching the narrow opening, gas pressure and speed increase, focusing the thin stream of liquid until it is so small only a single protein or virus can fit into each droplet.
In 2008 Spence and his colleagues, including Berkeley Lab’s David Shapiro, successfully tested the GDVN on the Advanced Light Source beamline 9.0.1, managed by Berkeley Lab’s Stefano Marchesini. The test was done with protein microcrystals extracted from the fluid in which researchers were attempting to grow larger crystals. These are the smallest protein nanocrystals from which diffraction patterns have ever been obtained, and the first from membrane protein nanocrystals – among the most resistant to crystallization.
Although the microcrystals weren’t individual protein specimens, and while the 9.0.1’s x-ray beams aren’t as bright or as rapidly pulsed as SLAC’s LCLS will be, the experiment demonstrated the jet technique’s high potential for speeds and exposures that won’t subject the samples to radiation damage. Some of the patterns the researchers obtained come from nanocrystals just a few molecules on a side, with a width of about 100 billionths of a meter (100 nanometers). At SLAC, the researchers plan to steadily reduce the nanocrystal size down to single molecules.
The corresponding reduction in scattered intensity will hasten and improve lensless imaging. The first step in lensless imaging is scattering the beam from the sample; the second step is constructing the image by interpreting and combining the data from the diffracted x-rays.
In order to merge the different views (projections) of an object, which is subsequently vaporized in this “diffract-and-destroy” mode, it is important that they all be identical. In biology, that leaves only molecules like proteins and viruses. DNA or RNA inside a virus is often packed differently in each virus, and cells are not identical at the molecular level, so cannot be studied in 3-D by this method.
Besides identical particles, successful data-merging also depends partly on knowing how the sample was oriented in the beam – easy to do with a large crystal, not so easy to do with a sample inside a drop of liquid whizzing across the beam. It may be possible to orient flying droplets by optical methods such as polarized laser beams or with specially shaped nozzles.
Perhaps simpler is to use the ever-increasing power of the computer – which for a lensless imaging system is where most of the functions of a lens reside. Computer systems have been developed that infer the orientation of the sample from the diffraction pattern itself, even when as few as four percent of the pixels in the detector light up. It does take a lot of diffraction patterns to derive an image this way – as many as 10 million – which will take the LCLS a few hours until better ways of orienting the droplets can be devised.
Nevertheless, Spence’s group recently obtained excellent diffraction patterns of MS2 virus capsids at the ALS by subtracting the diffraction “noise” of the liquid jet itself. These capsids, made in Mat Francis’s lab at the University of California at Berkeley, are the shells of the virus lacking its RNA genome and have the regular shape of buckyballs. Eventually the LCLS will be able to get a good diffraction pattern from a target like this with a single ultrabright pulse. In this case, however, computer processing was able to derive an excellent pattern by averaging diffraction from a series of samples.
DePonte will soon install Spence and Doak’s ultrafine, ultrafast “inkjet printer,” tested at the ALS, on the powerful new SLAC machine. It will be the first step into a bold new future for investigating the biological universe, one big molecule at a time.
Additional information
“X-ray imaging beyond the limits,” by Henry N. Chapman, appeared in Nature Materials, April, 2009.
“Powder diffraction from a continuous microjet of submicrometer protein crystals,” by D. A. Shapiro, H. N. Chapman, D. DePonte, R. B. Doak, P. Fromme, G. Hembree, M. Hunter, S. Marchesini, K. Schmidt, J. Spence, D. Starodub, and U. Weierstall, appeared in the Journal of Synchrotron Radiation, November, 2008.
“Gas dynamic virtual nozzle for generation of microscopic droplet streams,” by D. P. DePonte, U. Weierstall, K. Schmidt, J. Warner, D. Starodub, J. C. H. Spence, and R. B. Doak, appeared in the Journal of Physics D: Applied Physics, 19 September, 2008.