Contact: Lynn Yarris, (510) 486-5375, [email protected]
A new technique in Magnetic Resonance Imaging dubbed “Hyper-SAGE” has the potential to detect ultra low concentrations of clincal targets, such as lung and other cancers. Development of Hyper-SAGE was led by one of the world’s foremost authorities on MRI technology, Alexander Pines, a chemist who holds joint appointments with the Lawrence Berkeley National Laboratory (Berkeley Lab) and the University of California, Berkeley. The key to this technique is xenon gas that has been zapped with laser light to “hyperpolarize” the spins of its atomic nuclei so that most are pointing in the same direction.
“By detecting the MRI signal of dissolved hyperpolarized xenon after the xenon has been extracted back into the gas phase, we can boost the signal’s strength up to 10,000 times,” Pines says. “It is absolutely amazing because we’re looking at pure gas and can reconstruct the whole image of our target. With this degree of sensitivity, Hyper-SAGE becomes a highly promising tool for in vivo diagnostics and molecular imaging.”

Alex Pines (left), Xin Zhou and Dominic Graziani have created a new technique that boosts the signal strength of MRI and NMR spectroscopy up to 10,000 times. This enhanced sensitivity makes possible molecular imaging of clinical and environmental targets. (Photo by Roy Kaltschmidt, Berkeley Lab Public Affairs)
MRI is a painless and radiation-free means of obtaining high quality three-dimensional tomographical images of internal tissue and organs. It is especially valuable for optically opaque samples, such as blood. However, the application of MRI to biomedical samples has been limited by sensitivity issues. For the past three decades, Pines has led an on-going effort to find ways of enhancing the sensitivity of MRI and its sister technology, nuclear magnetic resonance (NMR) spectroscopy. Hyper-SAGE, the latest development, represents a significant new advance for both technologies, according to Xin Zhou, a member of Pines’ research group.
“Hyper-SAGE is a totally novel way to amplify a solvated xenon MRI/NMR signal in that instead of a chemical process, which is what previous signal enhancement techniques relied upon, it is a physical process,” says Zhou. “Because gas can be physically compressed, the density of information-carrying polarized gas in our detection chamber can be much greater than the density of an information-carrying solution. This means we can detect MRI signals from concentrations of molecules many thousands of times smaller than can be detected with conventional MRI.”
Zhou is the first author on a paper that is now available online in the Proceedings of the National Academy of Sciences (PNAS). The paper is entitled: “Hyperpolarized Xenon NMR and MRI Signal Amplification by Gas Extraction.” Co-authoring the paper with Zhou and Pines was Dominic Graziani. All hold joint appointments with Berkeley Lab’s Materials Sciences Division and UC Berkeley’s Chemistry Department, where Pines serves as the Glenn T. Seaborg Professor of Chemistry.
So Powerful and Yet so Weak
The great contradiction about MRI/NMR spectroscopy is that for being two of the most powerful tools we have today for studying the chemical composition and structure of a sample, they are based on a stunningly weak signal. Both depend upon atomic nuclei that have an unpaired proton or neutron. Such nuclei spin on an axis like miniature tops, giving rise to a magnetic moment – meaning the nuclei act like magnets with a north and south pole. When exposed to an external magnetic field, these spinning “bar magnets” attempt to align their axes along the lines of magnetic force. Since the alignment is not exact, the result is a wobbling rotation, or “precession,” that’s unique to each type of atom.
If, while exposed to the magnetic field, the precessing nuclei are also hit with a radiofrequency pulse, they will absorb and re-emit energy at specific frequencies according to their rate of precession (NMR). When the rf pulse is combined with magnetic field gradients a spatially encoded signal is produced that can be detected and translated into three-dimensional images (MRI).
Obtaining an MRI signal from a sample depends upon the spins of its precessing nuclei being polarized so that an excess point either “up” or “down.” MRI’s inherent weakness stems from the fact that the natural excess of up versus down spins for any typical population of atomic nuclei in a sample is only about one in 100,000. For this reason, conventional MRI techniques are designed to detect nuclei that are highly abundant in tissue, usually the protons in water. In addition, clinicians use contrasting agents to induce detectable changes in the MRI signal from a sample that can reveal the presence of anomalies. However, the sensitivity is usually too low for molecular imaging, which is needed in cancer detection, for example, where the earliest detections generally produce the most favorable outcomes.
Enter Hyper-SAGE
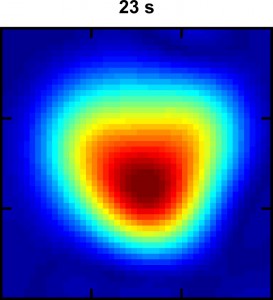
This Hyper-SAGE image of xenon dissolved in water flowing through a phantom lung shows the intensity of the MRI signal 23 seconds into the process. The warm colors (red, orange and yellow) represent a stronger signal than the cool colors. (image from Xin Zhou)
Pines and his research group have developed numerous ways of increasing the sensitivity of MRI technology and expanding its applicability. Previous work showed that xenon, an inert gas whose nuclei naturally feature a tiny degree of spin polarization, can be hyperpolarized with laser light to produce a population of xenon atoms in which nearly five out of every 10 nuclei – instead of one out of every 100,000 – produce an MRI signal. Pines and his group also showed that xenon can be incorporated into a biosensor and linked to specific proteins or other biological molecules to produce spatial images of a chosen molecular or cellular target.
The new technique, Hyper-SAGE, for “hyperpolarized xenon signal amplification by gas extraction,” offers other major advantages over conventional MRI/NMR techniques in addition to a signal that is up to 10,000 times stronger than previous signals, according to Zhou.
“Xenon gas has an intrinsically long relaxation time, greater than 45 minutes, which means the signal lasts long enough for us to collect all the encoded information, which in turn can enable us to detect specific targets, such as cancer-related proteins, at micromolar or parts per million concentrations,” he says. “Also, Hyper-SAGE utilizes remote detection, meaning the signal encoding and detection processes are physically separated and carried out independently. This is a plus for imaging the lung, for example, where the signal of interest would occupy only a small portion of the traditional MRI signal receiver.”
In their PNAS paper, Zhou, Graziani and Pines describe the successful testing of the Hyper-SAGE technique on a pair of membranes that mimicked the function of the lungs. Hyper-polarized xenon was dissolved in solution in one membrane to mimic inhalation, and was then extracted as a gas for detection from the other membrane to represent exhalation.
Explains Zhou, “In a clinical setting, a patient would inhale the hyperpolarized xenon gas which would be dissolved in the blood and allowed to flow into the body and brain. The exhaled xenon gas would then be collected and its MRI signal would be detected. Used in combination with a target-specific xenon biomolecular sensor, we should be able to study the gas-exchange in the lung and detect cancerous cells at their earliest stage of development.”
This research was supported by the U.S. Department of Energy’s Office of Science, through its Basic Energy Sciences programs.
Berkeley Lab is a U.S. Department of Energy national laboratory located in Berkeley, California. It conducts unclassified scientific research for DOE’s Office of Science and is managed by the University of California. Visit our Website at www.lbl.gov/
Additional Information
For more information about the research of Alexander Pines and his group, visit the Web at http://waugh.qb3.berkeley.edu/