Will we one day design and create molecules, cells and microorganisms that produce specific chemical products from simple, readily-available, inexpensive starting materials? Will the synthetic organic chemistry now used to produce pharmaceutical drugs, plastics and a host of other products eventually be surpassed by metabolic engineering as the mainstay of our chemical industries? Yes, according to Jay Keasling, chemical engineer and one of the world’s foremost practitioners of metabolic engineering.
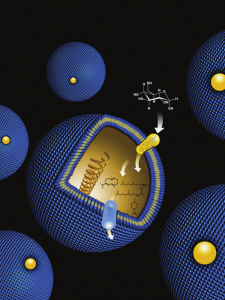
Metabolic engineering - the practice of altering genes and metabolic pathways within a cell or microorganism – could one day be used to mass-produce biofuels, pharmaceuticals and other chemical products from inexpensive and renewable starting materials. (Image by Flavio Robles, Berkeley Lab Public Affairs)
In a paper published in the journal Science titled “Manufacturing molecules through metabolic engineering,” Keasling discusses the potential of metabolic engineering – one of the principal techniques of modern biotechnology – for the microbial production of many of the chemicals that are currently derived from non-renewable resources or limited natural resources. Examples include, among a great many other possibilities, the replacement of gasoline and other transportation fuels with clean, green and renewable biofuels.
“Continued development of the tools of metabolic engineering will be necessary to expand the range of products that can be produced using biological systems, Keasling says. “However, when more of these tools are available, metabolic engineering should be just as powerful as synthetic organic chemistry, and together the two disciplines can greatly expand the number of chemical products available from renewable resources.”
Keasling is the chief executive officer for the Joint BioEnergy Institute, a U.S. Department of Energy (DOE) bioenergy research center. He also holds joint appointments with the Lawrence Berkeley National Laboratory (Berkeley Lab), where he oversees that institute’s biosciences research programs, and the University of California (UC), Berkeley, where he serves as director of the Synthetic Biology Engineering Research Center, and is the Hubbard Howe Jr. Distinguished Professor of Biochemical Engineering.
Metabolic engineering is the practice of altering genes and metabolic pathways within a cell or microorganism to increase its production of a specific substance. Keasling led one of the most successful efforts to date in the application of metabolic engineering, when he combined it with synthetic organic chemistry techniques to develop a microbial-based means of producing artemisinin, the most potent of all anti-malaria drugs. He and his research group at JBEI are now applying that same combination to the synthesis of liquid transportation fuels from lignocellulosic biomass. In all cases, the goal is to engineer microbes to perform as much of the chemistry required to produce a desired final product as possible.
“To date, microbial production of natural chemical products has been achieved by transferring product-specific enzymes or entire metabolic pathways from rare or genetically intractable organisms to those that can be readily engineered,” Keasling says. “Production of non-natural specialty chemicals, bulk chemicals, and fuels has been enabled by combining enzymes or pathways from different hosts into a single microorganism, and by engineering enzymes to have new function.”
These efforts have utilized well-known, industrial microorganisms, but future efforts, he says, may include designer molecules and cells that are tailor-made for the desired chemical and production process.

Jay Keasling, one of the world’s leading authorities on metabolic engineering, heads the Joint BioEnergy Institute and the Synthetic Biology Engineering Research Center. He also holds joint appointments with the Lawrence Berkeley National Laboratory and the University of California, Berkeley. (Photo by Roy Kaltschmidt, Berkeley Lab Public Affairs)
“In any future, metabolic engineering will soon rival and potentially eclipse synthetic organic chemistry,” Keasling says.
Keasling cites the production of active pharmaceutical ingredients as one area where metabolic engineering enjoys a distinct advantage over synthetic organic chemistry. This includes three specific classes of chemicals – alkaloids, which are primarily derived from plants; polyketides and non-ribosomal peptides, which are produced by various bacteria and fungi; and isoprenoids, which also are typically produced by microbes.
“Many of these natural products are too complex to be chemically synthesized and yet have a value that justifies the cost of developing a genetically engineered microorganism,” Keasling says. “The cost of starting materials is generally a small fraction of the complete cost of these products, and relatively little starting material is necessary so availability is not an issue.”
Keasling also says that metabolic engineering could provide a valuable alternative means of producing variations of terpenes, the hydrocarbon compounds common to the resins of conifers, in a form that could yield pharmaceuticals that are more effective for the treatment of human disease than the forms that nature has provided.
Perhaps the ripest targets of opportunity for future metabolic engineering efforts are petroleum-based bulk chemical products, including gasoline and other fuels, polymers and solvents. Because such products can be inexpensively catalyzed from petroleum, microbial production has until now been rare, but with fluctuating oil prices, dwindling resources and other considerations, the situation, Keasling says, has changed.
“It is now possible to consider production of these inexpensive bulk chemicals from low-cost starting materials, such as starch, sucrose, or cellulosic biomass with a microbial catalyst,” he says. “The key to producing these bulk chemicals in metabolically-engineered cells will be our ability to make the exact molecule needed for existing products rather than something ‘similar but green’ that will require extensive product testing before it can be used.”
In his Science paper, Keasling discusses the formidable roadblocks that stand in the way of a future in which microorganisms and molecules can be tailor-made through metabolic engineering, including the need for “debugging routines” that can find and fix errors in engineered cells. However, he is convinced these roadblocks can and will be overcome.
“One can even envision a day when cell manufacturing is done by different companies, each specializing in certain aspects of the synthesis, with one company constructing the chromosome, one company building the membrane and cell wall bag, and one company filling this bag with the basic molecules needed to boot up the cell.”
The Joint BioEnergy Institute (JBEI) is one of three Bioenergy Research Centers funded by the U.S. Department of Energy to advance the development of the next generation of biofuels. It is a scientific partnership led by Berkeley Lab and including the Sandia National Laboratories, the University of California campuses of Berkeley and Davis, the Carnegie Institution for Science, and the Lawrence Livermore National Laboratory.
The Synthetic Biology Engineering Research Center (SynBERC) is a multi-institution partnership, funded by the National Science Foundation, that is aimed at “making biology easier to engineer.” The SynBERC partnership is led by UC Berkeley and includes UC San Francisco, Harvard, MIT, Stanford, and Prairie View A&M University.
Berkeley Lab is a U.S. Department of Energy national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California for the DOE Office of Science. Visit our Website at www.lbl.gov/
Additional Information
For more information about the research group of Jay Keasling, visit the Website at http://keaslinglab.lbl.gov/wiki/index.php/Main_Page
For more information about JBEI, visit the Website at www.jbei.org
For more information about the DOE Bioenergy Research Centers visit the Website at http://genomicscience.energy.gov/
For more information about SynBERC visit the Website at http://www.synberc.org/