A few short decades ago, few could have imagined that the world would be seriously concerned over something called dysprosium. Also known as number 66 on the periodic table, dysprosium was once just another element for chemistry students to memorize but is now one of the most sought-after and critically needed materials on the planet.
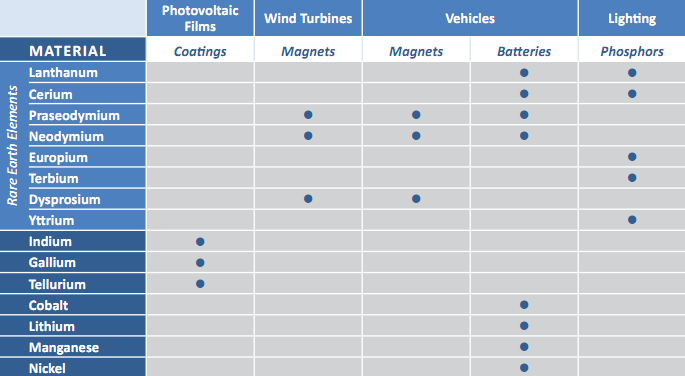
Belonging to a family of elements known as lanthanides—also called rare earths—dysprosium and other rare earths are used in almost every high-tech gadget and clean energy technology invented in the last 30 years, from smart phones to wind turbines to hybrid cars. Although the United States was self-sufficient in rare earths or obtained them on the free market until the early 2000s, the vast majority are now mined in China and the supply has been subject to fluctuations. The Department of Energy’s (DOE) Lawrence Berkeley National Laboratory (Berkeley Lab) aims to change the status quo by reviving the study of these critical materials to better understand how to extract them, use them more efficiently, reuse and recycle them and find substitutes for them.
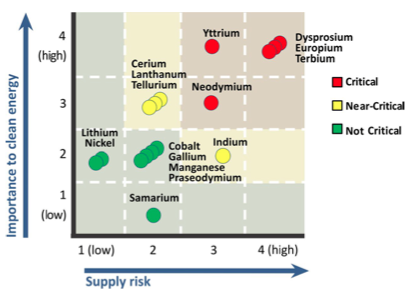
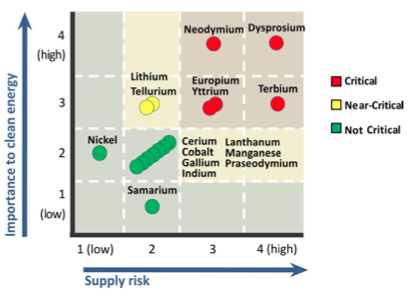
The top matrix shows the supply risk and importance to clean energy of certain elements in the short term (present-2015). The bottom matrix shows the medium term (2015-2025). (Source: DOE)
In its 2011 Critical Material Strategy released last month, the DOE said that “supply challenges for five rare earth metals (dysprosium, neodymium, terbium, europium and yttrium) may affect clean energy technology deployment in the years ahead.” It also recommended enhanced training of scientists and engineers to “address vulnerabilities and realize opportunities related to critical materials.”
“If we are going to achieve what we need to do in terms of managing climate change, we absolutely have to fix the materials problem—it’s the linchpin for clean energy technologies,” said Frances Houle, a Berkeley Lab scientist who is Director of Strategic Initiatives in the Chemical Sciences Division. “Because Berkeley Lab is such a broad institution, many of the pieces required are already here. We have the chemistry, the earth science, the materials science, the theory. Not very many institutions can say that.”
Like coal and gold, the rare earths are mined out of the ground. However, in any given ore, they are mixed together with other rare earths. So although they are not actually rare, they are difficult to mine. “They’re in low concentration, and it’s very hard to mine them and separate them out, so it’s challenging and extremely energy-intensive to produce rare earth materials ready for industrial manufacturers; it requires a lot of electricity, water and chemicals,” said Berkeley Lab Senior Scientist David Shuh. “This area of study has been ignored over the last two decades, largely due to insufficient research and development support.”
Shuh is the lead investigator on a Berkeley Lab project that takes a multidisciplinary approach to the issues, reinvigorating the study of the fundamental chemistry and materials sciences while taking advantage of advances in nanoscience, earth sciences, genomics and energy analysis techniques to devise innovative solutions.
While the United States has some scientists working in the rare earth field, China has at least 100 times as many. “The U.S. used to have the leadership in the chemistry and materials sciences of these materials, but now we are losing competitive advantages in these areas,” Shuh said. “We need to rev up rare earth science from top to bottom if we want to retain leadership in the use of these critical materials.”
Batteries, photovoltaics and lighting are just a few of the industries that could be crippled without reliable access to materials such as cerium, neodymium and terbium. Dysprosium is used in high-performance magnets (for cars, wind turbines, disc drives and a myriad of other uses) essential for the implementation of many clean energy technologies. In addition to the rare earths, there are a number of other so-called “energy critical elements” in other parts of the periodic table, including lithium, helium, cobalt and rhenium, that are crucial to a clean energy economy and are currently found in a limited number of places.
The resources devoted to studying the rare earths have not changed much since around the time the color television was invented. But in the meantime, their price has skyrocketed, increasing by nearly a factor of 1,000 in some cases, and scientists and engineers continue to rely on decades old science to address the energy challenge today. Moreover, new ways to use rare earths are being developed all the time.
More advanced study of the chemical and materials properties of the energy critical elements would not only aid in mining, separating, processing, and using them in current applications more efficiently but would also allow scientists to better understand—and thus find—substitutes for them. Plus, it should accelerate technological breakthroughs. “With better science, you’ll have better discovery and better technology,” Houle said. “It’s not feasible to go on a fishing expedition any more. You must have theory to guide the discovery effort.”
The Chemical Sciences Division of Berkeley Lab is world renowned in the study of actinides, a close neighbor of the lanthanides (rare earths) and which bear some chemical similarities. One goal of Shuh’s project is to improve understanding of their fundamental interactions by coupling theory to spectroscopic results, paving the way for the design of more efficient element-specific separations and development of new applications in fields such as lighting and biotechnology.
Complementing this approach, Berkeley Lab’s Materials Science Division will focus on basic research into understanding the properties of materials to come up with new alternatives that mimic those properties. “For example, certain wind turbines and motors rely on neodymium magnets. A better microscopic understanding may point toward new replacement materials containing elements that are more environmentally friendly or abundant,” said Jeff Neaton, deputy director of the division. “It may be that replacements draw on a combination of materials, a composite or assembly, or reduced dimensionality, as in nanostructures.”
Neaton added that recent advances in nanoscience, which allows researchers to synthesize and control materials at the level of atoms and molecules and a few tens of nanometers, has potential to play a large role in the process. Also, new nanoscale characterization tools and theory could bring breakthroughs in understanding that will be important in guiding the search for replacement materials.
Berkeley Lab’s Earth Sciences Division has deep experience in the modeling of subsurface chemical processes and in geochemical analysis of mineral surface structure and pore chemistry, expertise that will be useful in studying new ways to recover rare earth elements. Another approach would take advantage of “-omics” methods (which includes genomics and proteomics) to identify microorganisms that could aid in releasing rare earths from minerals.
At the other end of the process but encompassing the overall use of rare earth materials, researchers Jim McMahon and Eric Masanet of Berkeley Lab’s Environmental Energy Technologies Division specialize in analyzing industrial processes and quantifying the environmental and energy implications. Their lifecycle analysis of critical materials will focus on how to reuse and recycle them in efficient and environmentally acceptable ways.
Currently, the rare earth elements in computers, smart phones and other electronic gadgets are often either thrown away or sent abroad to be recovered—typically using low-cost labor and environmentally hazardous means. Today’s cell phones use 40 different elements; a Toyota Prius contains approximately 30 pounds of rare earth material.
“The materials are not designed to be easily recovered from the product, so we would look at the entire process of how something is manufactured, such as car batteries, and see if the battery can be designed and manufactured in a way to get the same performance but so that not only do we not waste anything but also puts the metal in a form that we can get it back,” said McMahon.
The analysis and modeling adds two other dimensions missing from many other lifecycle analyses: place and time. “If you look at a plant in California versus Wyoming, there’s different weather, different water availability, different pollutants, so it matters where you are,” McMahon said. “It also matters when you do it: things like photovoltaics are evolving, so five years from now, it will be different materials and different technologies.”
Many factors ranging from political events to environmental trends to changes in markets for products influence the availability of resources for manufactured goods. “A critical material today wasn’t a critical material 20 or 30 years ago,” Houle said. “Who knows what the crisis is going to be in 30 years. The main goal should be to be more resilient to shortages. Having alternatives and good reuse and recycling programs is essential.”
# # #
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.