
Water droplets in the morning mists of the Amazon jungle condense around aerosol particles. In turn, the aerosols condense around miniscule salt particles that are emitted by fungi and plants during the night. (Fabrice Marr, Creative Commons)
It’s morning, deep in the Amazon jungle. In the still air innumerable leaves glisten with moisture, and fog drifts through the trees. As the sun rises, clouds appear and float across the forest canopy … but where do they come from? Water vapor needs soluble particles to condense on. Airborne particles are the seeds of liquid droplets in fog, mist, and clouds.
To learn how aerosol particles form in the Amazon, Mary Gilles of the Chemical Sciences Division at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and David Kilcoyne of the Lab’s Advanced Light Source (ALS) worked with Christopher Pöhlker of Germany’s Max Planck Institute for Chemistry (MPIC) as part of an international team of scientists led by MPIC’s Meinrat Andreae and Ulrich Pöschl. They analyzed samples of naturally formed aerosols collected above the forest floor, deep in the rainforest.
Combined with results from other facilities, the ALS analysis provided essential clues to the evolution of fine particles around which Amazon clouds and fog condense, beginning with chemicals produced by living organisms. The team found that among the most important initial triggers of the process are potassium salts.
Dissecting invisible aerosols
At ALS beamline 5.3.3.2, the researchers performed scanning transmission x-ray microscopy (STXM) to determine the near-edge x-ray absorption fine structure (NEXAFS) of particles collected during the wet season in the remote, pristine forest northeast of Manaus, Brazil.
“Through absorption of soft x-rays by an atom’s core electrons, and subsequent emission of photons, the identity and exact location of the elements in the aerosol samples can be identified,” says Kilcoyne. “The essence of STXM is that it not only tells you if carbon is present but how this carbon is bound to other elements within the aerosol particles. This allows us to distinguish between soot, which is graphitic, and organic carbon.”
The researchers found three different types of organic aerosol particles, all similar to laboratory-generated reference samples: oxidation products based on precursor chemicals emitted in the gas phase by trees, including terpenes (the major component of turpentine) from tree resin, and isoprene, another organic compound abundantly released through leaves.
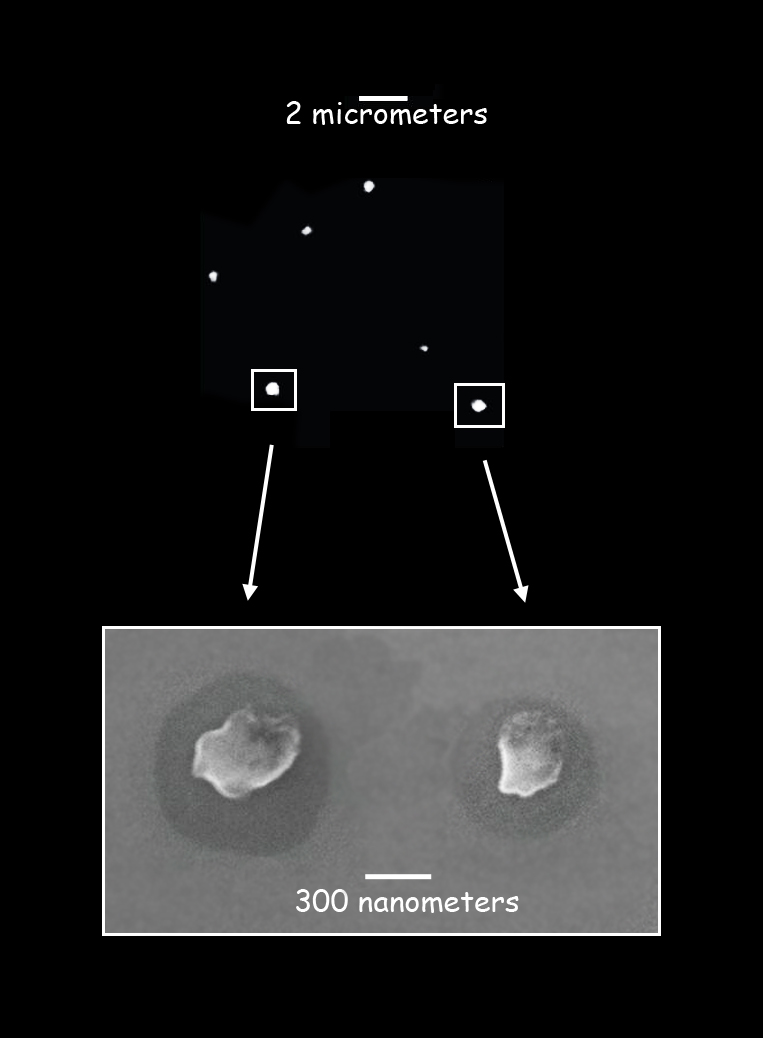
At top, STXM shows a bright potassium signal from small aerosol samples collected in the morning. Below, scanning electron microscope images show the organic material that has condensed around the potassium salt cores. Micrometers are millionths of a meter, and nanometers are billionths of a meter. (Advanced Light Source, Lawrence Berkeley National Laboratory, and Max Planck Institute for Chemistry)
“In the beginning we focused on the carbon, oxygen, and nitrogen contents of the organic materials,” says Pöhlker. “But then, to our surprise, we found very high potassium levels, up to 20 percent.” The 77 Amazonian aerosol samples were remarkable for the strong signal of potassium, in the form of salts, in all but three of them.
The samples were on the scale of mere millionths or billionths of a meter. The smaller the aerosol, the greater the proportion of potassium – those collected early in the morning were the smallest and richest in potassium. Larger particles contained more organic material but not more potassium. These facts suggest that potassium salts generated during the night acted as seeds for gas-phase products to condense onto, forming aerosols of different kinds.
“Biomass burning is also a rich source for potassium-containing aerosols in forested regions, but potassium from forest fires is correlated with the presence of soot, a graphitic form of carbon,” Gilles says. “Before and during the collection period there were no documented fires that could have affected the biosphere where the samples were collected, and no evidence of soot was observed in the samples. Hence the source of potassium could only have been natural forest organisms.”
Prime suspect
Fungal spores in the larger aerosol samples pointed to the prime suspect. Some fungi launch spores by building up water pressure through osmosis in sacs (asci) that contain the spores; when the pressure is great enough, the ascus bursts and squirts the spores into the air, along with fluid containing potassium, chloride, and sugar alcohol. Other fungi fire “ballistospores” when water vapor in the atmosphere condenses and causes a sudden release of restraining surface tension, also ejecting potassium, sodium, phosphates, sugars, and sugar alcohol.
Other biogenic mechanisms also release salts into the early morning mists that cover the forest, including salts dissolved in water by transpiration during the day and, at night, the oozing of sap rich in sugars, minerals, and potassium from the edges of leaves.
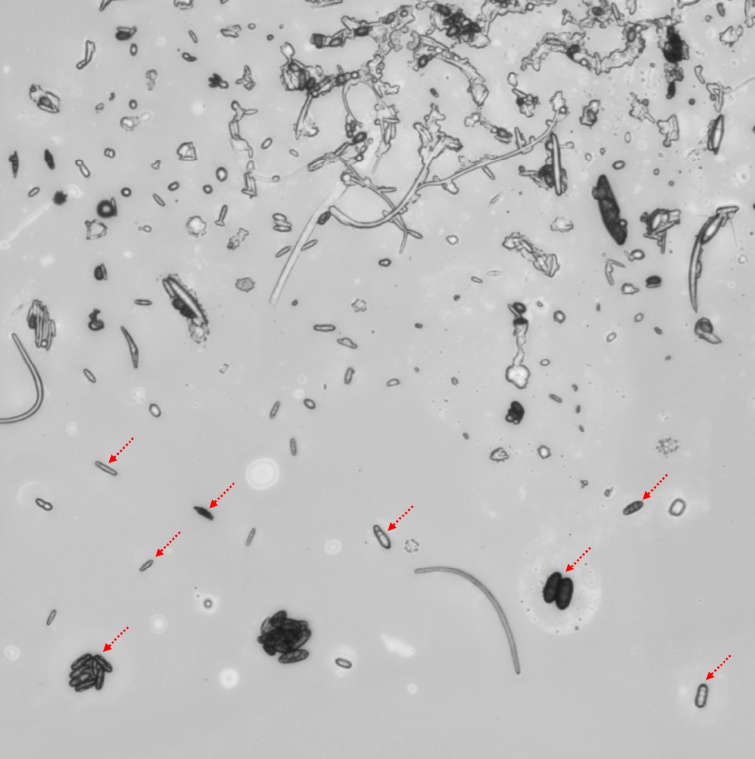
Under a light microscope, diverse and abundant fungal spores (red arrows) are visible in a large aerosol particle. (Max Planck Institute for Chemistry)
Thus invisibly tiny grains of potassium salts, generated by natural plants and other living things at night and early in the morning, play a key role in the formation of aerosols in the rainforest.
Terpenes and isoprenes are primarily released in the gas phase by plants in the jungle, and once in the atmosphere they react with water, oxygen, and organic compounds, acids, and other chemicals exuded by indigenous plants. These reaction products are less volatile and initiate the condensation within the low-lying forest biosphere. Since the smallest particles are typically the most important in condensation, potassium salts fill the role. As the day goes on, gas-phase products continue to condense and the particles continue to grow.
Throughout the rainy season the cloud cover, precipitation, water cycle, and finally the climate of the Amazon basin and beyond can be traced back to salts from fungi and plants in the undisturbed jungle, providing the precursors of natural cloud-condensation nuclei and directly influencing how fog and clouds form and evolve in the rainforest.
###
“Biogenic potassium salt particles as seeds for secondary organic aerosol in the Amazon,” by Christopher Pöhlker, Kenia T. Wiedemann, Bärbel Sinha, Manabu Shiraiwa, Sachin S. Gunthe, Mackenzie Smith, Hang Su, Paulo Artaxo, Qi Chen, Yafang Cheng, Wolfgang Elbert, Mary K. Gilles, Arthur L. D. Kilcoyne, Ryan C. Moffet, Markus Weigand, Scot T. Martin, Ulrich Pöschl, and Meinrat O. Andreae, appears in the August 31, 2012 issue of Science and is available online at http://www.sciencemag.org/content/337/6098/1075.abstract.
The Max Planck Institute for Chemistry’s press release on this research is at http://www.mpg.de/6329380/plants_fungi_salt-aerosol. Work at the Advanced Light Source was supported by DOE’s Office of Science.
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
The Advanced Light Source is a third-generation synchrotron light source producing light in the x-ray region of the spectrum that is a billion times brighter than the sun. A DOE national user facility, the ALS attracts scientists from around the world and supports its users in doing outstanding science in a safe environment. For more information visit www-als.lbl.gov/.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit the Office of Science website at science.energy.gov/.