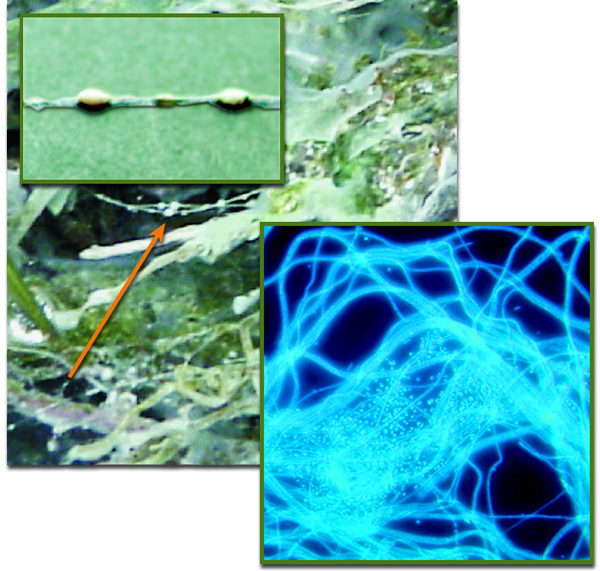
Strings of pearls (arrow and upper inset), whose "pearls" are up to three millimeters in diameter, were found where SM1 Euryarchaea live in close association with bacteria in the cold sulfidic streams of Germany’s Sippenauer Moor. Part of a pearl (lower inset) reveals colonies of microscopic spherical SM1 surrounded by filamentous bacteria. (Moissl-Eichinger group. Click on image for best resolution.)
In the fall of 2010, Hoi-Ying Holman of the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) was approached by an international team researching a mysterious microbial community discovered deep in cold sulfur springs in southern Germany.
“They told me what they were doing and said, ‘We know what you contributed to the oil-spill research,’” recalls Holman, who heads the Chemical Ecology group in Berkeley Lab’s Earth Sciences Division. “They wondered if I could help them determine the biochemistry of their microbe samples.”
Holman had co-authored a report in Science about bacteria in the Gulf of Mexico that thrived on the Deepwater Horizon oil plume. Using infrared spectromicroscopy at the Berkeley Synchrotron Infrared Structural Biology (BSISB) facility, which she directs at the Advanced Light Source (ALS), Holman helped determine how the novel bug obtained energy by eating the spilled crude. No stranger to subsurface bioscience, Holman would soon add a new actor to her cast of remarkable microbes.
Not extreme, but weird anyway
The name Archaea means “ancient things,” but Archaea were recognized as a distinct domain of life less than forty years ago. First thought to be exclusively extremophiles – lovers of boiling hot springs, deep-sea black smokers, acid mine runoff, and other inhospitable environments – more and more archaea are found thriving in moderate and cold environments, almost always as minority members of much larger microbial communities.
A unique exception to this pattern was discovered less than 10 years ago in the Sippenauer Moor in Germany. In microbial mats in this cold sulfur spring’s outflow, the SM1 Euryarchaeon lives in roughly equal abundance with bacteria in a community that forms symbiotic “strings of pearls”: the archaea fill the “pearls” and filamentous bacteria cover the pearl surfaces and form strings between them. The two kinds of microbes were assumed to be syntrophic – dependent on each other for nourishment – but the biochemical details were a mystery.
Christine Moissl-Eichinger of the University of Regensburg was among the SM1 Euryarchaeon’s discoverers. Before long what she calls “another amazing lifestyle” of the new archaeon emerged; biofilms that grew deep below the surface of another cold sulfur spring, the nearby Muehlbacher Schwefelquelle. Moissl-Eichinger and her team collected samples of the slime-like biofilm – which first seemed to be pure SM1 – on net traps underwater.
Microbial communities are essential to cleaning up subsurface pollution – including residues of metals and radionuclides at sites once involved in nuclear weapons research and assembly. Susan Hubbard heads the Earth Sciences Division’s (ESD’s) Environmental Remediation and Water Resources Program, with several projects that take advantage of synchrotron radiation Fourier-transform infrared spectromicroscopy – SR-FTIR for short – to understand biochemical changes in living microorganisms during the oxidation or reduction of uranium and chromium wastes.
SR-FTIR doesn’t require intrusive cell labeling, it can readily and nondestructively distinguish archaea from bacteria and assess their biochemistry, and it can follow changes in chemical composition of different members of the same microbial community over time. A mainstay of the Berkeley Synchrotron Infrared Structural Biology program (BSISB), the technology is increasingly recognized as an indispensable tool for microbiological investigation.
Microbial ecologist Eoin Brodie of ESD uses SR FTIR to understand how communities of soil microbes change as the climate changes, and how the composition of wood materials changes as they pass through a beetle’s gut. Meanwhile a team from halfway around the world is investigating how climate change affects crop growth in Australia, examining the “rizosphere,” the subsurface realm where symbiotic microorganisms extend from plant roots to collect water and nutrients such as nitrogen.
“At the BSISB we examine living root hairs in our microfluidic chamber, with just enough moisture to sustain life but not enough to absorb the infrared beam,” says its director Hoi-Ying Holman. “We can see how root growth is affected by introducing different microorganisms and watching the microbes and root hairs interact in real time.”
Examining life’s changing chemistry in real time isn’t confined to microorganisms. One example is wound healing, a research project by a team from the University of California at Davis and the University of Aberdeen in Scotland. Living human cells are imaged under the infrared beam to see what molecular changes are triggered in response to electric fields.
BSISB’s beamline 5.4 is unique at the Advanced Light Source. “Unlike x-rays, long-wavelength infrared reflects from ordinary mirrors and can be steered around sharp corners,” explains ALS infrared expert Michael Martin. “So instead of the crowded ALS floor, where x-ray beamlines are packed side by side, BSISB can spread out on its concrete-block roof.”
Extra room on the roof means live samples can be prepared in situ and studied with an array of imaging and spectroscopic techniques—an ideal facility for life sciences, environmental sciences, materials sciences, and a range of applications limited only by the users’ imaginations.
To augment their already extensive research, Moissl-Eichinger and Alexander Probst of her staff brought the Regensburg samples to Berkeley Lab, initially attracted by the PhyloChip, a DNA microarray invented by Berkeley Lab’s Gary Andersen and Todd DeSantis and their colleagues. Because the PhyloChip probes for the 16S rRNA gene, found in all Bacteria and Archaea, it can quickly and accurately sort through all known species in a sample – including those, like SM1 and many other microorganisms, that can’t be grown in culture.
Probst and DeSantis, both now with Second Genome, Inc., and Andersen were joined by Kasthuri Venkateswaran of the Jet Propulsion Laboratory, a member of NASA’s Biotechnology and Planetary Protection Group. Probst wanted to know who was living where in the subsurface sulfur-spring samples; Venkateswaran’s interest is understanding the role of Archaea in space and analogous sites. Although SM1 was by far the dominant species in the subsurface community, they found that small amounts of other archaea were present as well – and about five percent of the community consisted of bacteria.
Bring on the synchrotron
Led by Andersen, the PhyloChip’s inventors had contributed to the oil-spill research, and their previous association with Holman brought her and her BSISB colleagues aboard the SM1 research team.
“Lots of biochemical techniques can tell you what’s in a sample – lipids and carbohydrates, for example – but just because they’re there doesn’t mean they interact,” says Holman’s colleague Giovanni Birarda, a member of the BSISB staff. “Synchrotron radiation–based Fourier-transform infrared spectromicroscopy – SR-FTIR – takes images and spectra of the same sample, so you can map the chemical relationships by combining the images with spectra that identify where the archaea and bacteria are.”
Holman says, “The main difference is in their membrane lipids. Bacterial membrane lipids consist of fatty acids with long alkylic chains” – functional groups of singly bonded carbon and hydrogen atoms – “which have only one to two terminal methyl groups,” groups with one carbon and three hydrogen. “By contrast, archaeal membrane lipids generally consist of branched and saturated isoprenes” – a more complex common hydrocarbon – “and are relatively less alkylic but have more methyl groups.”
By revealing the bright spectral signals of alkylic and methyl groups, together with sulfur functional groups, synchrotron FTIR unambiguously identified the sulfate-reducing metabolic activity of the bacteria within the SM1 samples. The archaeal cells themselves showed no such activity, leading the researchers to posit a thriving mutual metabolism of the archaea and bacteria.
In many cases, such syntrophy requires close physical association. Covering the surface of each SM1 cell the researchers found spines made of three protein strands, equipped with terminal hooks where the strands divided. Moissl-Eichinger named them hami, Latin for barbs or hooks. These “nano-grappling hooks” apparently hold the microbial partners together, working in synchronization. The major hami protein is unlike any known proteinaceous archaeal or bacterial filaments.
How SM1 Euryarchaea interact with their bacterial partners may be a model for understanding other syntrophic relations essential to the carbon and sulfur cycles on which Earth’s life depends. So far found in just two sites in Germany, the species is the only example yet of an archaeon that dominates a biological ecosystem – but related species have been found in sulfur springs as far afield as Turkey and may be widespread.
DOE’s Office of Science supported building and equipping the BSISB and also supports the ALS.
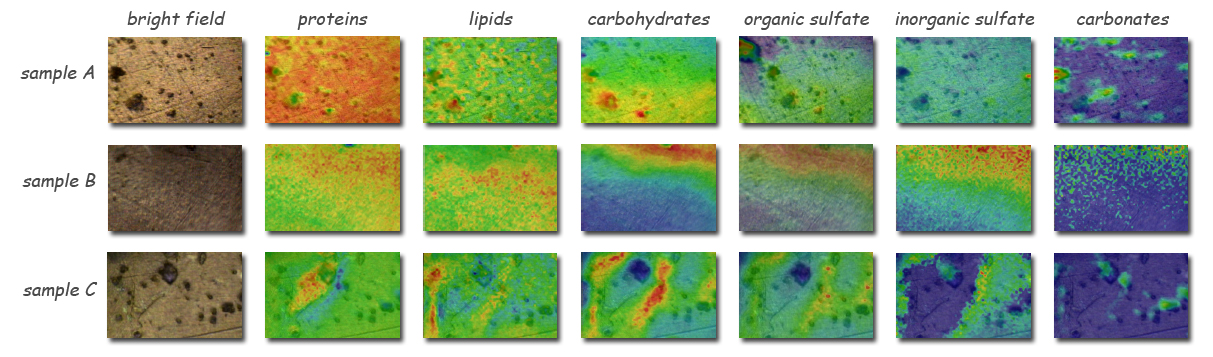
Fourier-transform spectromicroscopy of three samples of archaea-dominated biofilms from the Muehlbacher Schwefelquelle, using the Berkeley Synchrotron Infrared Structural Biology facility’s bright infrared beams, differentiates between archaea and bacteria by their lipid and carbohydrate signatures and shows that organic sulfates and carbonated minerals are located with the bacteria. (BSISB. Click on image for best resolution.)
###
“Tackling the minority: sulfate-reducing bacteria in an archaea-dominated subsurface biofilm,” by Alexander J. Probst, Hoi-Ying N. Holman, Todd Z. DeSantis, Gary L. Andersen, Giovanni Birarda, Hans A. Bechtel, Yvette M. Piceno, Maria Sonnleitner, Kasthuri Venkateswaran, and Christine Moissl-Eichinger, appears in advanced online publication of The ISME Journal, 22 November 2012, and is available at http://www.nature.com/ismej/journal/vaop/ncurrent/abs/ismej2012133a.html.
The Science article on novel hydrocarbon-degrading bacteria associated with the Deepwater Horizon oil spill may be found at http://www.sciencemag.org/content/330/6001/204.abstract.
More about the Berkeley Synchrotron Infrared Structural Biology Program is at http://infrared.als.lbl.gov/content/structuralbiology/overview.
For Susan Hubbard’s research, including bioremediation of uranium-contaminated sites, see http://esd.lbl.gov/about/staff/susanhubbard/ and http://esd.lbl.gov/research/programs/erwr/
Eoin Brodie’s microbial research is described at http://envmicro.wordpress.com/
More about how climate change may affect microbial communities important to crop growth in Australia is at http://www.csiro.au/en/Organisation-Structure/Divisions/Plant-Industry/michellewatt.aspx
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit the Office of Science website at science.energy.gov/.