Ana Correia (foreground) with Mina Bissell showed that the protein MMP3 promotes invasion through its hemopexin domain by extracellular interaction with the protein HSP90b. (Photo by Roy Kaltschmidt, Berkeley Lab)
New evidence supports earlier findings that cancer therapy drugs based on a family of enzymes called metalloproteinases (MMPs) failed in clinical trials because they were aimed at the wrong target. Berkeley Lab researchers who previously demonstrated that MMP14 interacts with a partner to promote mammary invasion and – under abnormal conditions – tumors through a mechanism distinct from catalytic activity, have now shown that MMP3 can also promote tumors but via interaction with a different partner.
“Taken together results of these two studies provide a compelling explanation for why inhibitors of MMPs failed so dramatically in clinical trials,” says Mina Bissell, Distinguished Scientist with Berkeley Lab’s Life Sciences Division and one of the world’s foremost breast cancer authorities who led both studies. “Targeting non-catalytic MMP sites and MMP-interacting partners with agents such as small inhibitors or antibodies could yield more effective and tissue-specific cancer inhibitors.”
For girls at the onset of puberty, breast tissue begins to morph into milk glands. In this process, called branching morphogenesis, mammary epithelial cells migrate outward into the fat pad to form a tree of milk ducts. Although an invasive act, branching morphogenesis is normally a precise and quantitative process involving a complex interplay of both intracellular and extracellular matrix signals. Should normal signaling be disrupted, however, branching morphogenesis can lend itself to the development of breast cancer.
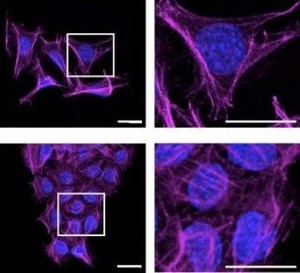
MMP3 hemopexin domain induces altered morphology and invasion in mammary epithelial cells. Shown here are filamentous actin proteins (magenta) and breast cell nuclei (blue) in culture.
MMPs are crucial to the development of healthy tissue architecture and play critical roles in numerous physiological and pathological processes. However, the over-expression of many MMPs, including MMP14 and MMP3, are known to promote the growth of tumors. In the case of breast cancer, the over-expression of MMPs has been strongly linked to early patient deaths. Consequently, pharmaceutical companies have sought to develop breast cancer therapies by inhibiting MMP catalytic activity, which was believed to be the only activity triggered by MMPs.
“We have now shown in two separate experimental models and projects by two different teams that while MMPs may use their catalytic domains to clear a path for cells to invade, the signaling that causes cancer to develop is due to non-catalytic domains,” Bissell says.
In the earlier study of mouse mammary cells, Bissell, working with Hidetoshi Mori and others in her research group, showed that MMP14, a membrane-bound protein, promotes mammary invasion and branching through its association with the beta 1 subunit of integrin (Itgb1), a signaling molecule involved in branching and fat pad invasion. Now, working with Ana Correia and others, Bissell has shown that MMP3, a secreted protein, promotes invasion through its hemopexin domain by extracellular interaction with the heat shock protein HSP90b.
“MMP3 binds to HSP90b, a very important protein that also is misregulated or overexpressed in cancer,” Correia says. “The big surprise is that HSP90b, which is responsible for the correct folding of the proteins inside the cell, has a crucial role outside the cell to regulate MMP3-driven invasion. Blocking of extracellular HSP90b with inhibitory antibodies added to the medium abolished invasion and branching”.
Calling these findings “dramatic and novel,” Bissell says, “The two experiments show that our understanding of how MMPs as a family of related proteins function is at best incomplete if not incorrect in terms of signaling to their targets.”
Working with Bissell and Correia on this latest study were Hidetoshi Mori, Emily Chen and Fernando Schmitt.
Additional Information
For more on Bissell’s breast cancer research go here