
An expedition into the Luquillo Experimental Forest in Puerto Rico by JBEI and Berkeley Lab researchers led to the identification of a soil microbe that utilizes lignin as its sole source of carbon.
Nature designed lignin, the tough woody polymer in the walls of plant cells, to bind and protect the cellulose sugars that plants use for energy. For this reason, lignin is a major challenge for those who would extract those same plant sugars and use them to make advanced biofuels. As part of their search for economic ways to overcome the lignin challenge, researchers at the Joint BioEnergy Institute (JBEI) have characterized the enzymatic activity of a rain forest microbe that breaks down lignin essentially by breathing it.
“Using a combination of transcriptomics and proteomics we observed the anaerobe Enterobacter lignolyticus SCF1 as it grows on lignin,” says Blake Simmons, a chemical engineer who heads JBEI’s Deconstruction Division. “We detected significant lignin degradation over time by absorbance, suggesting that enzymes in E. lignolyticus could be used to deconstruct lignin and improve biofuels production. Our results also demonstrate the value of a multi-omics approach for providing insight into the natural processes of bacterial lignin decomposition.”
Not only does lignin inhibit access to cellulose, the by-products of lignin degradation can also be toxic to microbes employed to ferment sugars into fuels. This makes finding microbes that can tolerate a lignin environment a priority for biofuels research. Tropical rainforests harbor anaerobic microbes that actually utilize lignin as their sole source of carbon. Kristen DeAngelis, a microbial ecologist formerly of JBEI and now with the University of Massachusetts, has led expeditions to the Luquillo Experimental Forest where she and her crew harvested soil microbes.
“Tropical soil microbes are responsible for the nearly complete decomposition of leaf plant litter in as little as eighteen months,” she says. “The fast growth, high efficiency and specificity of enzymes employed in the anaerobic litter deconstruction carried out by these tropical soil bacteria make them useful templates for improving biofuel production.”
In an earlier study at JBEI led by DeAngelis, E. lignolyticus SCF1 is a member, was shown to be capable of anaerobic lignin degradation, but the enzymes behind this degradation were unknown. Through their multi-omics approach plus measurements of enzyme activities, DeAngelis, Simmons and their colleagues were able to characterize the mechanisms by which E. lignolyticus SCF1 is able to degrade lignin during anaerobic growth conditions.
“We found that E. lignolyticus SCF1 is capable of degrading 56-percent of the lignin under anaerobic conditions within 48 hours, with increased cell abundance in lignin-amended compared to unamended growth,” Simmons says. “Proteomics analysis enabled us to identify 229 proteins that were significantly differentially abundant between the lignin-amended and unamended growth conditions. Of these, 127 proteins were at least two-fold up-regulated in the presence of lignin.”
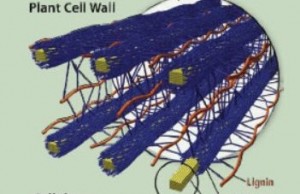
The lignin in plant cell walls that helps protect energy-storing sugars must be degraded for cost-effective production of advanced biofuels.
This new study also showed that E. lignolyticus SCF1 is able to degrade lignin via both assimilatory and dissimilatory pathways, the first soil bacterium to demonstrate this dual capability.
“Our next step is to look at what kind of chemical bonds are preferred by these two different pathways of reduction,” DeAngelis says. “We can then try to develop tailored routes to targeted intermediates by defining the molecular mechanisms of enzymatic reactions with lignin.”
This work was supported by the University of Massachusetts, Amherst, the Environmental Molecular Sciences Laboratory (EMSL) and JBEI through the U.S. Department of Energy’s Office of Science. A paper describing this research has been published in the journal Frontiers in Microbiology (Microbial Physiology and Metabolism) under the title “Evidence supporting dissimilatory and assimilatory lignin degradation in Enterobacter lignolyticus SCF1.” In addition to DeAngelis and Simmons, other authors were Deepak Sharma, Rebecca Varney, Nancy Isern, Lye Markillie, Carrie Nicora, Angela Norbeck, Ronald Taylor, Joshua Aldrich and Errol Robinson.
Additional Information
For more about the Joint BioEnergy Institute go here
For more about Kristen DeAngelis go here
For more about the Environmental Molecular Sciences Laboratory go here
