Contact: Dan Krotz, (510) 486-4019, [email protected]
There’s a new clue in the search to identify the harbingers of Alzheimer’s disease.
Memory loss in cognitively normal elderly people may in some cases be related to the accumulation of a plaque in the brain that is implicated in Alzheimer’s disease, as well as damage to a part of the brain that is involved in memory function, according to a recent study led by scientists from Berkeley Lab and the University of California, Berkeley.
The scientists believe this cluster of events could signal the earliest stages of the disease.
Efforts to predict who is likely to develop Alzheimer’s disease, before symptoms develop, have gained importance in recent years as pharmaceutical companies work to develop preventative drug therapies. Some drugs in development are designed to block the deposition of beta-amyloid protein in the brain. Beta-amyloid is the main constituent of a plaque that plays a central role in Alzheimer’s disease.
“Because of the promise of these therapies, we want to identify people who are at really high risk for the disease, but don’t have symptoms yet,” says William Jagust, a staff scientist in Berkeley Lab’s Life Sciences Division who also has appointments at UC Berkeley’s School of Public Health and the Helen Wills Neuroscience Institute. Jagust conducted the study, which was recently published online in the journal Brain, with UC Berkeley graduate student Elizabeth Mormino and scientists from several other institutions.
The team found that the more beta-amyloid plaque in a person’s brain, the more the person is likely to have a smaller hippocampus, which is the part of the brain that forms new memories. The person is also more likely have impaired episodic memory, which is the type of memory that enables someone to recall events tied to a specific time and place, such as what they had for breakfast, or where they left their car keys.
Not everyone who has deposits of amyloid plaque in their brain develops Alzheimer’s disease. The same goes for people with spotty episodic memory. But Jagust believes the confluence of three signs of Alzheimer’s disease — beta-amyloid deposition, hippocampus atrophy, and episodic memory loss — fits a modus operandi that is difficult to ignore.
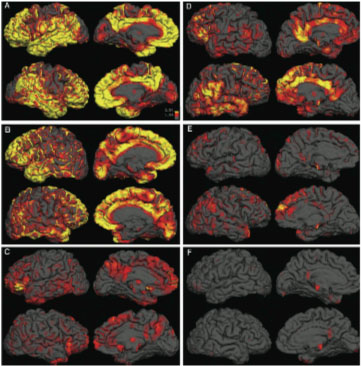
Alzheimer's disease on the horizon? PET scans revealed beta-amyloid plaque in the brains of three Alzheimer's disease patients (left) and three normal controls (right). The yellow indicates high uptake of a label that targets beta-amyloid plaque, and the red indicates medium uptake.
“These changes in older people are not necessarily benign. When you start to cluster them together, it begins to sound like Alzheimer’s disease,” says Jagust. “And this suggests that some of the changes we are detecting in normal older people may be indicative of the subsequent development of Alzheimer’s.”
The team recruited 20 elderly residents in Berkeley, CA who have no signs of cognitive impairment. They then evaluated these volunteers using state-of-the-art imaging tools at Berkeley Lab.
Positron emission tomography (PET) was used to measure the presence of amyloid plaque in the volunteers’ brains. The PET scans utilized a groundbreaking radioactive label that targets beta-amyloid plaque so that it can be detected on a scan. The label, called PIB, was developed in 2004 by University of Pittsburgh scientists and allows researchers to image the presence of amyloid plaque in patients’ brains. Before, such measurements could only be conducted at autopsy. Magnetic resonance imaging was used to measure hippocampus volume. A memory test gauged the volunteers’ episodic memory.
The scientists found that the signatures of Alzheimer’s disease came in packages of three. A person with increased beta-amyloid plaque is more likely to have a shrunken hippocampus and poorer episodic memory. The results are consistent with a widely held model in which amyloid deposition damages the hippocampus, which in turn saps episodic memory. Now, these events has been glimpsed in normal elderly people.
“They are cognitively normal. We wouldn’t say they have they have Alzheimer’s by any stretch of the imagination,” says Jagust. “But the fact that these things occur together makes us consider the possibility that this is a very early change in the brain that could be leading to Alzheimer’s disease.”
The team replicated these results in a similar group of normal elderly people, and in a group of people with mild cognitive impairment, by analyzing information collected in a national database of brain scans called the Alzheimer’s Disease Neuroimaging Initiative.
The research was funded by the National Institutes of Health and the Alzheimer’s Disease Neuroimaging Initiative.
Additional Information:
- Learn more about Jagust’s research.