Contact: Lynn Yarris (510) 486-5375, [email protected]
“If we want to make a big hit at changing the greenhouse gas situation and related environmental problems, we have to recognize the fact that in many cases we’re off by a large factor from where we need to be and yet we’re in a hurry,” said Paul Alivisatos, interim Director of Berkeley Lab and the Larry and Diane Bock professor of Nanotechnology at UC Berkeley, speaking at last week’s annual meeting of the American Academy for the Advancement of Sciences (AAAS), which was held in Chicago.
“We need to think hard about reconnecting basic and applied research in many areas of renewable energy technologies because the solutions that we’re seeking involve fundamental problems that we don’t completely understand.”
National laboratories such as Berkeley Lab can serve as important “anchor points,” he said, working closely with universities and private companies to provide an institutional setting where basic and applied research interactions can take place in a timely way that will help the nation move forward on energy. He cited the Joint BioEnergy Institute (JBEI) and other U.S. Department of Energy Bioenergy Research Centers as the models for going “from science to solutions.” The goal is to integrate basic scientific research into scaled-up applied technologies.
“I think the national laboratories are going to play an incredibly important role as the nation enters into a mode of trying to go after the energy problem in a new way,” Alivisatos said. “To be successful, we in the national laboratory system will need to work with each other and with our colleagues in universities and private companies.”
Alivisatos was a panel member at a symposium entitled “Basic Research for Global Energy Security: A Call to Action.” His talk was titled: Nanoscale Materials for Solar Fuel Generation. In this talk he described the efforts by a broad collation of scientists at Berkeley Lab and UC Berkeley to develop artificial nanoscale materials that could be used to convert the energy of sunlight into electricity or store it in a transportable fuel.
The U.S. currently consumes about 3.4 terawatts of energy a year, which is the equivalent of 34 billion 100-watt light bulbs burning constantly. At present, the bulk of this energy is derived from non-renewable fossil fuels (oil, gasoline, coal and natural gas) that, when burned, add carbon to the earth’s atmosphere, which is a major factor behind global warming. The demand for energy is expected to grow upwards of 50 percent for electricity alone by the year 2030, making the need to find renewable and carbon-neutral alternative energy sources imperative. Photovoltaic or solar cells hold enormous promise for helping to meet this need, but currently the cost per kilowatt hour of electricity produced by these devices is prohibitive by a factor of at least five.
“Although photovoltaics cells based on inorganic single crystals are well-established, new phenomena on the nanoscale, coupled with the possibility of new fabrication methods, suggest that solutions to the cost factor could be found in nanoscale photovoltaics,” Alivisatos said. “Solar cell materials that are as abundant but much less costly than silicon and other semiconductors in use today could substantially reduce the cost per kilowatt hour.”
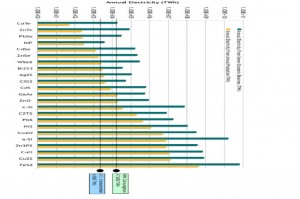
A study by Paul Alivisatos, Cyrus Wadia and Daniel Kammen identified iron pyrite and copper oxide as the two materials with the greatest potential in terms of cost and abundance for use in photovoltaics. (Click to enlarge)
In his talk, Alivisatos described a study of potential photovoltaic materials that he undertook with Cyrus Wadia, a member of his research group, and Daniel Kammen, UC Berkeley professor of energy and resources and director of the Renewable and Appropriate Energy Laboratory. Currently, photovoltaic cells are made from either silicon or from thin films of cadmium telluride or copper indium gallium selenide. These materials can meet performance requirements – the power efficiency of a typical crystalline silicon cell is about 22 to 24 percent (meaning it converts up to 24 percent of the light striking it into electricity). However, these materials are too expensive to be scaled up to commercial scale – terawatt hours – energy production.
“To meet today’s energy demands, we need to be able to cover about 58 million acres of land with photovoltaics,” Alivisatos said. “This is about the amount of U.S. cropland that now sits idle.”
Usage on such a scale would quickly wipe out our supplies of current photovoltaic materials, so alternative materials must be found. In their study of potential new photovoltaic materials, Alivisatos and his colleagues identified iron pyrite and copper oxide as the two materials with the greatest potential in terms of cost and abundance. However, there are fundamental challenges that must be met in order to achieve adequate performance with high-speed assembly methods that could be scaled to large areas.
Alivisatos described a number of possible approaches to meeting these challenges including the use of dot and rod or wire-shaped nanocrystals and the use of polymer-nanocrystal hybrids that would simultaneously optimize light absorption, charge separation, charge transport and collection.
“Such hybrids use a minimum amount of material in a really cheap and scalable process,” he said.
He also described Berkeley Lab’s “Helios Artificial Photosynthesis Project,” in which the goal is to create photovoltaic cells in the form of a membrane of nanocrystals that would mimic photosynthesis, the process by which green plants convert sunlight into electrochemical energy.
“If we were to cover 58 million acres of non-arable land at various locations with photovoltaic nanocrystals that operated at a conversion efficiency of even one-percent, we could generate enough energy to replace all gasoline consumption in the U.S.,” said Alivisatos. “If we could reach a conversion efficiency of 10-percent we would far exceed the total energy consumption in this country today.”
Additional Information:
For more on Paul Alivisatos’ AAAS talk, read the UC Berkeley story at http://www.berkeley.edu/news/media/releases/2009/02/20_aaassolar.shtml
For more information about the research of Paul Alivisatos, visit the Website at http://www.cchem.berkeley.edu/pagrp/
For more information about the Helios Project, visit the Website at http://www.lbl.gov/msd/helios_site/index_helios.html
