Scientific contact: Hiroshi Imanaka, 520-621-7984
Most life can’t use stable nitrogen molecules unless they’re fixed in reactive compounds, so nitrogenated organic compounds are essential constituents of life on Earth and probably any other planet with life. While almost 80 percent of Earth’s atmosphere is nitrogen gas, only small amounts of fixed nitrogen are found there; most nitrogen compounds are currently made by life itself. How did the cycle begin?
Hiroshi Imanaka, a chemist and planetologist at the University of Arizona (UA) and, not incidentally, a member of the SETI Institute (SETI stands for Search for Extraterrestrial Intelligence), thinks the clues may lie in the atmosphere of Saturn’s largest moon, Titan. Titan has the only substantial atmosphere in our solar system besides Earth’s that’s mostly nitrogen.
“One of the most surprising results from NASA’s Cassini mission to the Saturn system was the recent discovery of large, complex organic molecules in Titan’s upper atmosphere,” Imanaka says.
Researchers suspect that the orange smog that gives Titan its color is made up mostly of hydrocarbon soot. “Whether it contains reactive nitrogen compounds is still a mystery,” says Imanaka, noting that such compounds might be precursors of life after they’ve settled on the moon’s surface. “If we can find processes that form such compounds in the upper atmosphere abiotically” – without the participation of life – “they might give us a clue to how life started on Earth.”
Short of hitching a ride on a passing spaceship, how does one study the atmosphere of Titan? The answer is to use synchrotron light from Berkeley Lab’s Advanced Light Source (ALS), optimized to produce bright beams of just the kind of extreme ultraviolet (EUV) and vacuum ultraviolet (VUV) light thought to energize Titan’s nitrogen chemistry.
At the ALS Chemical Dynamics Beamline 9.0.2, Imanaka and his UA colleague Mark Smith irradiated a mixture of nitrogen gas and methane in a 95-to-five-percent ratio, seeking to create highly reactive compounds capable of kick-starting the formation of nitrogenated organic aerosols in Titan’s atmosphere.
Ultraviolet experiments with carbon-nitrogen compounds found in Earth’s stratosphere have been conducted in the past, but the nitrogen-methane mix used by Imanaka and Smith was never tested this way. The researchers sent the mixture flowing through the synchrotron’s beam inside a vacuum chamber.
In initial runs, Imanaka and Smith used a mass spectrometer to analyze only the altered gases. They found mixtures of hydrocarbons like benzene in amounts consistent with what is actually observed in Titan’s atmosphere, but they saw no evidence of complex nitrogen compounds.
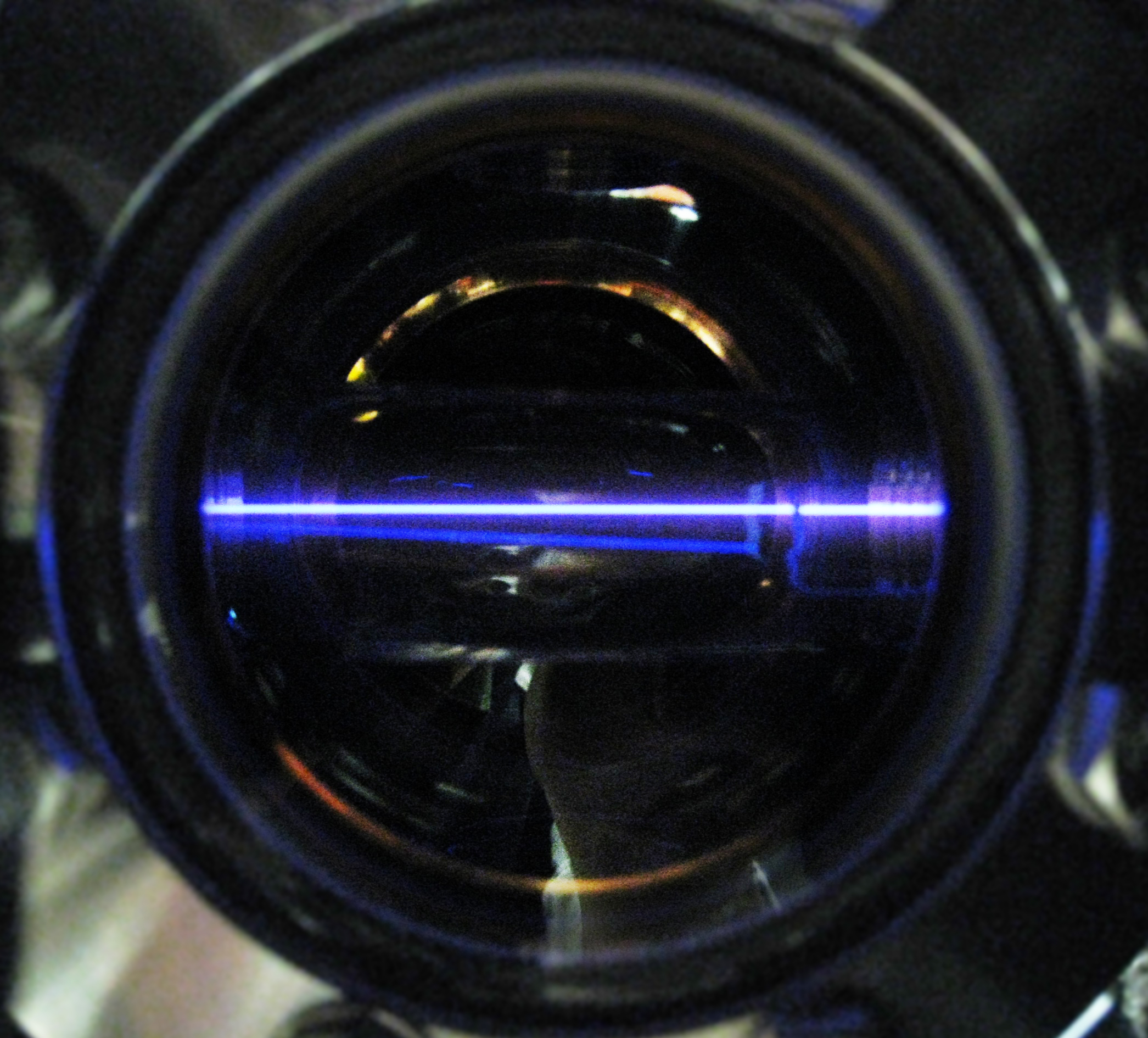
Nitrogen gas stimulated by the synchrotron beam glows inside a stainless steel vacuum chamber. ALS users Hiroshi Imanaka and Mark Smith brought their experimental equipment from the University of Arizona and assembled it at beamline 9.0.2.
The EUV-VUV beam can break apart a substantial number of nitrogen molecules (N2) into highly excited nitrogen atoms; this dissociated nitrogen should subsequently join with methane and methane products to produce an array of highly reactive nitrogenated species. The question is, says Imanaka, “Where did they go?”
Imanaka and Smith finally found the reactive nitrogenated species, stocked as complex nitrogenated organics, when they performed sophisticated mass spectrometry on deposits of brown gunk that had solidified on the wall of the vacuum chamber.
“Using synchrotron light we were able to present the first experimental demonstration of how EUV-VUV light modifies N2 molecules to convert complex organic macromolecules, under conditions much closer to the real atmosphere of Titan than previous research,” says Imanaka. “By detailed analysis of both gas and solid products, we found that dissociated nitrogen preferentially ends up in solid products through very reactive, short-lived, nitrogenated species.”
Is the brown gunk Titan smog? Chances are good that it’s at least the first step in a process that may make life possible on Titan and may have been an important early step toward life on Earth.
Additional information
“Formation of nitrogenated organic aerosols in the Titan upper atmosphere,” by Hiroshi Imanaka and Mark Smith, appears in the early online edition of Proceedings of the National Academy of Sciences for the week of 5 July 2010. Read the University of Arizona press release.
