A blustery nor’easter storm dumped heavy rain throughout most of the American Chemical Society’s Fall 2010 national meeting and exposition, which was held in Boston, August 22-26. However, the soggy weather did little to dampen the enthusiasm of the thousands of attendees. Although the theme of the meeting was “Chemistry for Preventing and Combating Disease” the presentations at this 240th national ACS meeting ranged far and wide across the broad chemical sciences spectrum. Berkeley Lab researchers contributed nearly 100 presentations to this mix, with particular emphasis on the areas of medical applications, in keeping with Fall meeting theme, plus applications in energy and the environment. Over the next few days, the Berkeley Lab News Center will highlight presentations from all three of these areas, starting with medical.
Berkeley Lab at ACS Fall Meeting 2010: Medical Applications
Chemistry of the Brain
 Christopher Chang, with Berkeley Lab’s Chemical Sciences Division, drew an especially large audience for his address at the Arthur C. Cope Scholars Award Symposium. In a talk titled “Molecular imaging approaches to understanding chemistry in the brain,” he discussed the enormous potential of hydrogen peroxide as a molecular imaging marker for understanding chemistry in the brain.”
Christopher Chang, with Berkeley Lab’s Chemical Sciences Division, drew an especially large audience for his address at the Arthur C. Cope Scholars Award Symposium. In a talk titled “Molecular imaging approaches to understanding chemistry in the brain,” he discussed the enormous potential of hydrogen peroxide as a molecular imaging marker for understanding chemistry in the brain.”
“The human brain is a unique chemical system that offers an incredibly rich opportunity for biochemistry studies,” Chang said. “For example, while the brain accounts for only about two-percent of human body mass, it is responsible for about 20-percent of total oxygen consumption, making it the body’s most oxidatively active organ under normal physiological conditions.”
Miss-regulation of oxygen at the cellular level, however, is connected to oxidative stress and damage that occurs with aging and age-related neurodegenerative diseases. Molecular imaging has the potential to provide new understanding of brain cell signaling, growth, and differentiation, which in turn could help in the development of new therapeutics for stroke and such neurodegenerative diseases as Alzheimer’s and Parkinson’s, Chang said.
To this end, Chang and his research group have been working to detect hydrogen peroxide, a simple but highly reactive molecule that is found throughout the brain. Hydrogen peroxide has been considered potentially damaging to cells through free radical chemistry, but the work of Chang and his group has shown that it can serve as a highly effective signaling agent. They’ve developed a series of hydrogen peroxide fluorescent tags that glow in specific colors and enable them to track small-molecule oxygen metabolites in living cells, tissue and organisms.
“We can use these hydrogen peroxide probes to study the complex roles of redox biology in brain health, aging and disease,” Chang said.
In their most recent work, Chang and his group were able to show that the water channel Aquaporin-3 (AQP3) facilitates the uptake of hydrogen peroxide into mammalian cells and mediates downstream intracellular signaling. This process until now has been poorly understood, but Chang and his group demonstrated that by tightly controlling the access of hydrogen peroxide to the cell, AQP3 helps regulate the molecule’s downstream intracellular effects.
“Our discovery shows that even though hydrogen peroxide is a potentially toxic molecule, its controlled use has beneficial physiological functions,” Chang said.
Macromolecular drug delivery
The use of modified virus capsids – a capsid is the protein shell of a virus – as targeted drug delivery vehicles was the subject of two Berkeley Lab presentations. One, given by Nicholas Stephanopoulos of the Materials Sciences Division and a member of the Matt Francis research group, described the use of the bacteriophage MS2 as a therapy vehicle for the treatment of Jurkat leukemia T cells. Jurkat cells are an immortalized line of T lymphocytes often used to evaluate the susceptibility of cancers to drug and radiation therapies.
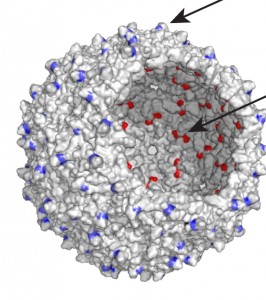 “Virus capsids make for excellent drug delivery vehicles because you can attach targeting molecules to the outside of the capsid and to the inside you can attach a drug molecule or multiple drug molecules,” Stephanopoulos said. “This gives you a delivery vehicle with a well-defined three-dimensional shape that allows for multivalent effects. It also provides you with modular and flexible modification strategies.”
“Virus capsids make for excellent drug delivery vehicles because you can attach targeting molecules to the outside of the capsid and to the inside you can attach a drug molecule or multiple drug molecules,” Stephanopoulos said. “This gives you a delivery vehicle with a well-defined three-dimensional shape that allows for multivalent effects. It also provides you with modular and flexible modification strategies.”
The bacteriophage MS2, which is an icosahedral virus of the Escherichia coli bacterium, is a particularly attractive nanoscale scaffold, Stephanopoulos said. MS2 can be expressed recombinantly in Escherichia coli where it spontaneously assembles into a non-infectious hollow sphere 27 nanometers in diameter, with 32 holes, two nanometers in diameter, for easy access to the capsid’s interior.
For their virus capsid delivery vehicles, Stephanopoulos and his collaborators in the Francis group use aptamers – segments of single-stranded DNA or RNA molecules – that have evolved to bind to specific types of cells. Easy to synthesize and functionalize, aptamers can be developed to bind to virtually any specific target, Stephanopoulos said. In the case of his ACS presentation, an aptamer was evolved to bind to Jurkat leukemia T cells. The interior of the capsid, once the virus genome was removed, was stuffed with up to 180 maleimide porphyrins capable of generating hundreds of thousands of biologically destructive singlet oxygen molecules upon illumination.
“These capsids were able to kill 76-percent of the Jurkat cells after only 20 minutes of illumination,” Stephanopoulos said. “The modified capsids were also able to kill Jurkat cells selectively even when mixed with erythrocytes. This suggests the possibility of using our system in a dialysis-based treatment that can target blood-borne cancers.”
Another member of the Francis research group, Wesley Wu, described the effective use of modified bacteriophage MS2 capsids to deliver taxol, a highly potent anti-cancer drug, in a talk titled “Genome-free viral capsids as multivalent carriers for taxol delivery.”
In this study, a water-soluble derivative of taxol was synthesized and attached to the interior surface of empty capsids of MS2. The modified capsids retained their form upon taxol attachment, as well as after extended incubation in cell culture media. When incubated with MCF-7 breast cancer cells, the capsids released taxol, and the resulting cell viability levels were similar to those observed upon treatment with free taxol in solution.
“In combination with available methods for the installation of peptide and aptamer-based targeting groups on the external surface of the MS2capsids, our attachment of taxol to the inside of the capsid could provide a new series of drug carriers that could be directed to a variety of specific tissue types,” Wu said.
Soft Contact Lenses and Bacteria
As those who wear “soft” contact lenses are painfully aware, without proper care these silicone hydrogel materials can become infected with bacteria. The most common invader is Pseudomonas aeruginosa, a pathogen that can cause microbial keratitis, an inflammation of the cornea that in severe cases can lead to loss of vision.
Victoria Tran, a member of the research group of Clayton Radke, a faculty researcher with Berkeley Lab’s Earth Sciences Division, gave a presentation titled “Strain and surface dependant bacterial attachment of Pseudomonas aeruginosa to contact lens materials.” In this talk, she discussed a new investigation into the critical question: How is Pseudomonas aeruginosa able to attach itself to soft contact lenses?
“Prevention of Pseudomonas aeruginosa attachment to soft-contact lenses may help reduce the risk of microbial keratitis, but there has been too much uncertainty regarding the attachment process, and the role of lens surface chemistry and strain variations on bacteria-lens interaction,” Tran said. “We tested the hypothesis that negatively charged polyelectrolyte hydrogels impact both Pseudomonas aeruginosa surface interaction and binding.”
The results of their investigation, Tran said, suggest that surface modification of contact lenses can have strain-dependent effects on Pseudomonas aeruginosa approach and attachment. These differences likely reflect variations in attachment strategies used by the bacteria, such as flagella, pili and lipopolysacharides, even within the same species.
“Our findings highlight the complexities involved in designing a universal strategy for reducing bacterial attachment to contact lenses and other surfaces,” Tran said.
Cholesterol and Membranes
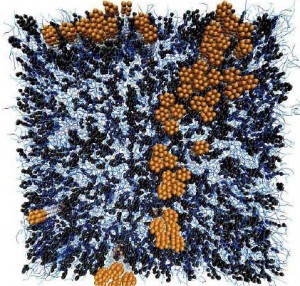 The cells in your body are Surrounded by an outer covering called the “plasma membrane” that consists of a continuous double-layer of phospholipids, embedded with proteins and cholesterol. The cholesterol helps maintain the structural integrity of the membrane and also facilitates cell signaling.
The cells in your body are Surrounded by an outer covering called the “plasma membrane” that consists of a continuous double-layer of phospholipids, embedded with proteins and cholesterol. The cholesterol helps maintain the structural integrity of the membrane and also facilitates cell signaling.
“Understanding the effect of cholesterol on the properties of membranes,” was the title of a presentation by Berend Smit, a faculty scientist with Berkeley Lab’s Materials Sciences Division and a leading authority on molecular simulations.
Molecules such as proteins and cholesterol in a membrane exist in a very different environment from molecules in the cell. To understand how proteins and cholesterol interact in a membrane, Smits and his research group have developed multi-scale computational models that allow them to study the effects of changes in the membrane structure on the collective behavior of these molecules.
In his ACS talk, Smit presented a “coarse-grained model of a hydrated saturated phospholipid bilayer containing cholesterol that we studied using a hybrid dissipative particle dynamics – Monte Carlo method.”
This approach, he said, “Allows us to reach the time and length scales necessary to study structural and mechanical changes of the bilayer at various temperatures and cholesterol concentrations.”
Properties studied by Smit and his collaborators included the area per lipid, condensation, bilayer thickness, tail order parameters, bending modulus and area compressibility.
“Our model quantitatively reproduces most of the experimental effects of cholesterol on these properties, and reproduces the main features of the experimental phase diagrams,” Smit said. “We also produced all-atom simulations of the system, which we used to further validate the structure of our coarse-grained bilayer.”
The model and simulations showed that adding cholesterol to a membrane causes the membrane to condense and also causes proteins in the membrane to cluster.
“Because changes in the structure of the membrane may have important consequences for the functioning of proteins, it is important to have a better molecular understanding of the cholesterol-induced changes,” Smit said.