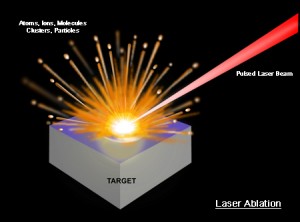
LAMIS uses the energy of a high-powered laser beam to ablate a tiny spot on a sample, creating a plasma plume for spectroscopic analysis that reveals chemical elements and their isotopes. (Image courtesy of Applied Spectra, Inc.)
At some point this year, after NASA’s rover Curiosity has landed on Mars, a laser will fire a beam of infrared light at a rock or soil sample. This will “ablate” or vaporize a microgram-sized piece of the target, generating a plume of ionized gas or plasma, which will be analyzed by spectrometers to identify the target’s constituent elements. Future Mars rovers, however, will be able to do even more. Researchers with the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab), in collaboration with Applied Spectra, Inc., have developed an advanced version of this laser technology that can also analyze a target’s constituent isotopes. This expanded capability will enable future rovers for the first time to precisely date the geological age of Martian samples.
Rick Russo, a scientist with Berkeley Lab’s Environmental Energy Technologies Division and a pioneer in laser ablation spectroscopy, led the development of LAMIS – for Laser Ablation Molecular Isotopic Spectrometry. As with the earlier Laser Induced Breakdown Spectroscopy (LIBS) technology being used on rover Curiosity, the basic premise is to use the energy of a high-powered laser beam focused to a tiny spot on the surface of a sample to create a plasma plume for analysis. Each species of atoms or ions within the plasma will emit light with signature spectral emission peaks. However, whereas LIBS only measures the optical emission spectra of atoms and ions, LAMIS measures the emission spectra of molecules and molecular ions. This enables LAMIS to identify the specific isotopes of a chemical element within the plasma plume.
“Relative to atomic emission, molecular spectra can exhibit significantly larger isotopic shifts due to the contributions of the vibrational and rotational motion in the molecule,” Russo says. “The trick is to be patient and wait for the hot atoms and ions in the plasma to collide and merge with the ambient environment to form an oxide, or a nitride or fluoride, and then collect the molecular light emissions.”

From left, Alexander Bol'shakov, Xianglei Mao and Rick Russo are part of the research team that developed LAMIS, a green chemistry laser spectroscopy technology that can be operated across vast distances. (Photo by Roy Kaltschmidt, Berkeley Lab)
Russo and his research group have been using LAMIS to study isotopes of strontium, an alkaline earth metal commonly found in geological and natural materials. Although strontium’s major isotopes are stable (strontium-90 being a notable exception), the percentage of strontium-87 will naturally increase over time as a result of the decay of radioactive rubidium. Comparing the ratio of strontium-87 to strontium-86 is a standard tool for age dating in geochronology, oceanography and archeology. The ratio of these strontium isotopes is also used to date the origin of historic or forensic samples. Currently, the standard means of measuring strontium isotopic ratios is by mass spectrometry technologies that involve time-consuming, labor-intensive laboratory sample dissolution work with an extensive array of instrumentation. This sample dissolution work generates substantial chemical waste. LAMIS offers a green chemistry alternative that is faster, less expensive and can be carried out from across vast distances.
“LAMIS is not yet as sensitive or precise as mass spectrometry but unlike mass spectrometry it does not require chemical dissolution sample preparation, vacuum chambers and a laboratory infrastructure,” Russo says. “All we need is a laser beam and an optical spectrometer and we can perform real-time isotopic analyses of samples at ambient pressures and temperatures.”

Artist’s concept of Rover Curiosity with LIBS technology firing a beam of infrared light at Martian rock for spectroscopic analysis. (Image courtesy of NASA)
LAMIS represents what may be the only practical means of determining the geochronology of samples on Mars or other celestial bodies in the Solar System. Current age estimates of such bodies suffer from uncertainties in the billions of years. That said, LAMIS also has many important applications here on Earth. Strontium isotope ratios have been a focus in the field of medicine for both treatment and diagnostic purposes. Measuring these ratios can also provide valuable information about atmospheric chemistry. They also can be used to trace the origins and movements of early humans. But perhaps the most immediate and important application of LAMIS will be in nuclear forensics aimed at non-proliferation and terrorism.
“Uranium and plutonium, like every chemical element, has a spectral signature that’s as unique as every human’s DNA or fingerprint,” Russo says. “With LAMIS, we can factor in isotopic ratios, giving us an additional level of identification that could be critical.”
For example, “yellow cake,” the powdered concentrate made from uranium ore that is a main ingredient of nuclear fuel, can also be used to fabricate a nuclear weapon. Measuring the elemental composition of yellow cake is one way of identifying the geographic locale where the yellow cake was produced, but because uranium ore is ubiquitous to our planet’s surface, being able to also measure isotopic ratios in a sample of yellow cake can be a huge advantage for pinpointing where it originally came from.
“The natural ratio of uranium-235 to uranium-238 is defined by the geology of our planet,” Russo says. “If you find a modified ratio in a sample then you know someone has been enriching that uranium. Other isotopic ratios within a nuclear reaction chain also can tell you how a nuclear weapon was made and where it might have originated.”
Much of this research was done in collaboration with Applied Spectra, a company Russo created in 2004 with the help of Small Business Innovation Research grants, to bring laser ablation spectroscopy technology to the marketplace.
“The next step is to improve the sensitivity and precision of LAMIS,” Russo says. “Our immediate target is parts-per-million, which should be relatively easy for us to reach, but ultimately we want to get to parts-per-billion sensitivity, which will be a challenge. However, 50 years ago, the parts-per-billion sensitivity of today’s mass spectrometry technologies would have been thought impossible.”
Russo and his colleagues have described their work on LAMIS in several papers, including one in which the technique was shown to be effective for measuring isotopes of boron. The most recent paper appeared in the journal Spectrochimica Acta Part B. The paper is titled “Laser Ablation Molecular Isotopic Spectrometry: Strontium and its isotopes.” Co-authoring this paper were Xianglei Mao, Alexander Bol’shakov, Inhee Choi, Christopher McKay, Dale Perry and Osman Sorkhabi. Co-author Bol’shakov with Applied Spectra, says his company is eager to commercialize this technology.
“We envision multiple applications for LAMIS in industry, medical diagnostics, nuclear safeguarding and other areas,” he says.
Support for this research came from the Defense Threat Reduction Administration of the U. S. Department of Defense, DOE’s National Nuclear Security Administration, and NASA through Applied Spectra, Inc.
# # #
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
Additional Information
For more information about the research of Rick Russo and his group visit the Website at http://teamd.lbl.gov/
For more information about Applied Spectra, Inc., visit the Website at http://www.appliedspectra.com/