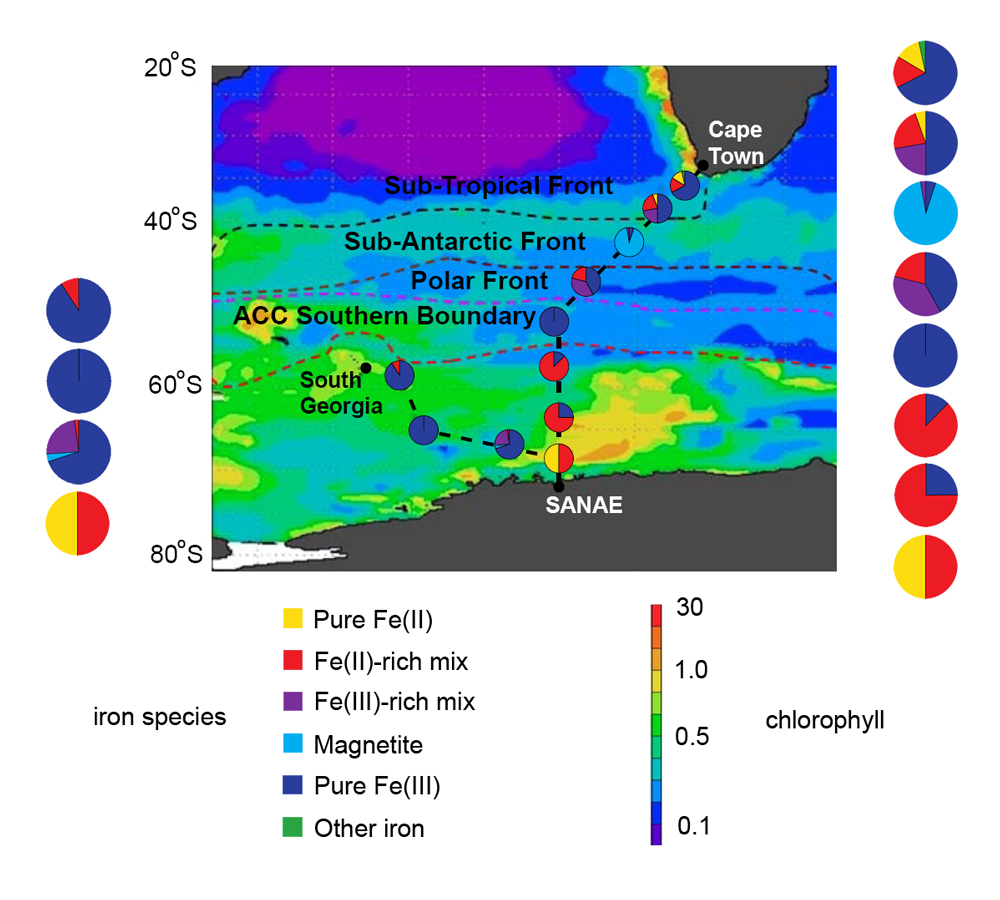
At bottom left, the kinds of iron species found in two transects of the Southern Ocean are shown in descending order from most soluble (yellow and red) to least soluble (purple and blue). The pie charts at right show the proportions of each species sampled at points between the SANAE base and Cape Town, and the pie charts at left show the samples between SANAE and South Georgia Island. (ACC stands for Antarctic Circumpolar Current.) The map shows chlorophyll concentrations in milligrams per square meter, per the scale at bottom right. There is a rough tendency for soluble iron regions to show greater chlorophyll concentrations. (Click on image for best resolution.)
The Southern Ocean, circling the Earth between Antarctica and the southernmost regions of Africa, South America, and Australia, is notorious for its High Nutrient, Low Chlorophyl zones, areas otherwise rich in nutrients but poor in essential iron. Sea life is less abundant in these regions because the growth of phytoplankton, the marine plants that form the base of the food chain, is suppressed. A study by scientists from South Africa’s Stellenbosch University, Princeton University, and Berkeley Lab’s Advanced Light Source (ALS) suggests the problem is not just a lack of iron but a lack of iron in easy-to-use form.
The researchers sampled two north-south corridors across the Southern Ocean, traveling an easterly transect between the base of the South African National Antarctic Expeditions (SANAE IV) in Queen Maud Land and Cape Town, and a westerly transect between SANAE IV and South Georgia Island. Along the way they collected particles countaining solid iron from a series of ocean systems with different characteristics.
The samples were analyzed at the ALS Molecular Environmental Sciences beamline 11.0.2 by team member Tolek Tyliszczak, using scanning transmission X-ray microscopy (STXM). STXM combines microscopy with spectroscopy, allowing the researchers to distinguish a number of different iron species and compounds in particles of different shapes and sizes. In this way the research team could determine the solubility of the iron particles and how easily they could be taken up by plankton or other life.
The most common forms of iron are Fe(II) and Fe(III). There’s plenty of highly oxidizing Fe(III) in sea water but it’s insoluble, and life has developed special mechanisms to absorb it. Fe (II) is readily soluble and easily taken up by plants and bacteria but sparse in sea water, except when freshly dumped there by continental run-off or wind-blown dust. Most dust is in the form of magnetite, however, a compound of both Fe(II) and Fe(III).
The researchers tested their particles for pure Fe(II), pure Fe(III), mixtures of these two with other materials (including aluminum), and magnetite. They found that the iron solubulity of their samples ranged over three orders of magnitude. Then they compared the predominant kinds of iron in a region with the pattern of summertime chlorophyll, that is, phytoplankton growth.
In the eastern transect, iron in the soluble Fe(II) form, either pure or dominant in a mixture, occurred relatively near the coasts of Africa and Antarctica. The regions of open ocean in between were dominated by Fe(III) or magnetite. The soluble Fe(II) regions clearly showed more growth of phytoplankton.
The westward transect was less cooperative: although the entire area was within the circumpolar current, close to the continental shelf of Antarctica, most of the iron on that leg was less soluble. Nevertheless, the team concluded that the abundance and distribution of more soluble forms of iron “reveals trends that allude to the effect of Fe speciation on biology, and vice versa.”
For more about this work, visit http://www.sciencemag.org/content/338/6111/1199.abstract.