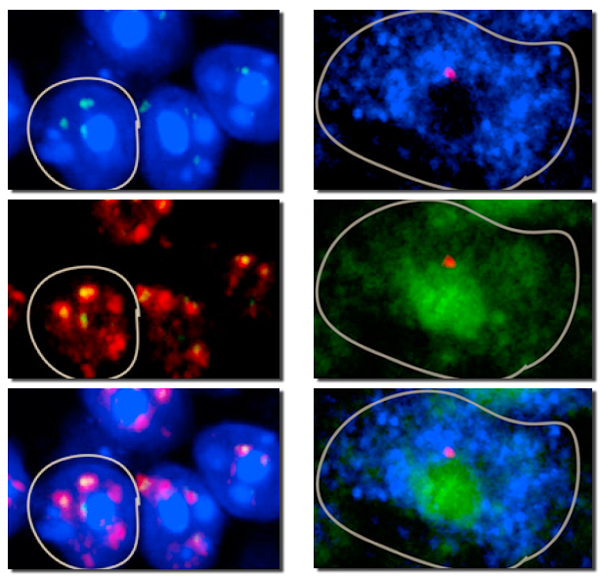
At left, the nucleus of a mouse olfactory sensory neuron (white outline) shows concentrations of dense heterochromatin surrounded by a handful of loci where silent olfactory receptor (OR) genes are sequestered. At right, the cell’s single active OR gene resides near the silent gene loci, but in euchromatin, not heterochromatin. (Different colors indicate different staining techniques.) (Images by Lomvardas lab)
Olfactory sensory neurons – nerve cells in the nose – directly sense molecules that convey scent, then send the signals to the brain. Biologists have long wondered how it’s possible for each nerve cell to be equipped with only one kind of olfactory receptor (OR). There are over a thousand different kinds of OR genes in humans, distributed widely over both chromosomes; mice, dogs, and other animals have many more.
A nerve cell’s initial choice of a specific allele, or form of the gene, is likely probabilistic. Once the choice is made, the challenge is to explain how the chosen allele is locked in: how does the cell silence the competing genes? The answer lies with an epigenetic mechanism – one that works outside genetic processes – to spatially rearrange the chromosomes.
Carolyn Larabell and Mark LeGros of Berkeley Lab’s Physical Biosciences Division and UC San Francisco were part of a team led by Stavros Lomvardas of UCSF’s Department of Anatomy to investigate OR expression in mice, which have some 2,800 different alleles of the OR gene.
Using a variety of stains and labeling techniques, Lomvardas and his colleagues found that almost all the OR genes were collected in a handful of focal points, or foci, near the center of the olfactory cell’s nucleus and on the borders of a dense region of heterochromatin, which contains relatively few genes. The OR genes in these loci were silent.
The single chosen OR alleles were located very near the dense silent regions, but across the border in gene-rich euchromatin territory, where they were readily expressed.
X-ray tomography of the nucleus of a wild-type olfactory sensory neuron shows the central position of the dense heterochromatin, where silent OR loci are found.
The reduced expression of a particular protein called the lamin b receptor proved crucial to creating this spatial sequestration of all but one OR gene. In other kinds of cells, the lamin b receptor anchors heterochromatin to the envelope of the cell’s nucleus. In mature olfactory sensory neurons, however, the heterochromatin is in the nucleus’s center.
To discover how the mechanism worked, Larabell and LeGros created 3D images of the olfactory neurons at the Advanced Light Source’s National Center for X-Ray Tomography (NCXT). X-ray tomography images whole hydrated cells that have not been fixed or stained, yielding 3D views that can be examined inside and out.
In wild-type olfactory neurons, with lamin b receptor expression suppressed, x-ray tomography revealed condensed chromatin structures corresponding to the foci where silent OR genes were concentrated in the central heterochromatin.
X-ray tomography of a mutant olfactory sensory neuron underlines the critical role of the lamin b receptor, which when expressed causes the nucleus to swell and its membrane to fold, and causes the dense central chromatin to disperse to the nuclear envelope. (Videos by National Center for X-ray Tomography)
For more on the epigenetic regulation of olfactory receptor expression, and the contributions of x-ray tomography to understanding its mechanism, see “Nuclear aggregation of olfactory genes governs their monogenic expression” in the journal Cell, at http://www.cell.com/abstract/S0092-8674%2812%2901286-X?switch=standard.
For more about the National Center for X-Ray Tomography, visit http://ncxt.lbl.gov/.