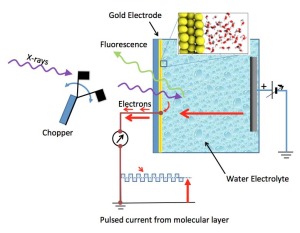
Schematic of the electrochemical cell – a silicon nitride (Si3N4) membrane separates the liquid from vacuum region of the x-ray source; a 20nm thin-film gold electrode is deposited on liquid side of the membrane. Detection of x-ray absorption is via fluorescence emission on the vacuum side or electron emission at the gold electrode.
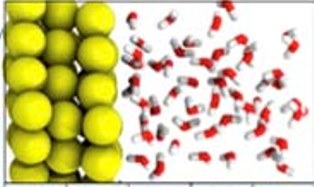
When a solid material is immersed in a liquid, the liquid immediately next to its surface differs from that of the bulk liquid at the molecular level. This interfacial layer is critical to our understanding of a diverse set of phenomena from biology to materials science. When the solid surface is charged, just like an electrode in a working battery, it can drive further changes in the interfacial liquid. However, elucidating the molecular structure at the solid-liquid interface under these conditions has proven difficult.
Now, for the first time, researchers at the US Department of Energy’s (DOE)’s Lawrence Berkeley National Laboratory (Berkeley Lab) have observed the molecular structure of liquid water at a gold surface under different charging conditions.
Miquel Salmeron, a senior scientist in Berkeley Lab’s Materials Sciences Division (MSD) and professor in UC Berkeley’s Materials Science and Engineering Department, explains this in the context of a battery. “At an electrode surface, the build-up of electrical charge, driven by a potential difference (or voltage), produces a strong electric field that drives molecular rearrangements in the electrolyte next to the electrode.”
Berkeley Lab researchers have developed a method not only to look at the molecules next to the electrode surface, but to determine their arrangement changes depending on the voltage. With gold as a chemically inert electrode, and slightly-saline water as an electrolyte, Salmeron and colleagues used a new twist on x-ray absorption spectroscopy (XAS) to probe the interface and show how the interfacial molecules are arranged.
Spewing photons and electrons
XAS itself is not new. In this process, a material absorbs x-ray photons at a specific rate as a function of photon energy. A plot of the absorption intensity as a function of energy is referred to as a spectrum which, like a fingerprint, is characteristic of a given material molecule and its chemical state. Our eyes recognize many materials by their characteristic colors, which are related to their visible light absorption spectra. The x-ray photons used in this study have energies that are about 250 times higher than those of visible light and are generated at Berkeley Lab’s Advanced Light Source (ALS).
Typical XAS measurements are made under vacuum conditions, as x-rays are readily absorbed by matter, even the nitrogen molecules in air. But liquids will quickly evaporate in a vacuum. By using a very thin (100 nm, or a tenth of a micrometer) x-ray transparent window, with a thin coating of gold (20nm), on a sealed liquid sample holder, the Berkeley Lab team was able to expose water molecules in the liquid to x-rays and collect their spectra.
(From left) Miquel Salmeron, Jinghua Guo, David Prendergast, Liwen Wan, Chenghao Wu, Tod Pascal and Juan-Jesus Velasco-Velez (Photo by Roy Kaltschmidt)
Upon absorbing an x-ray photon, the excited water molecule can spew (emit) either charged particles (electrons) or light (photons). The amount of photon emission, or fluorescence, is one indicator of how many x-ray photons have been absorbed. However, fluorescing x-rays can be detected from molecules ranging from those at the gold surface to those deep (micrometers) inside the liquid far from the influence of the gold surface, and these dominate the measured spectrum.
“We are only really interested in a nanoscale interfacial region, and looking at the fluorescence photon signal we can’t tell the difference between the interface and the interior electrolyte molecules,” says Salmeron.
The challenge therefore was to collect a signal that would be dominated by the interfacial region. The team accomplished this by measuring electron emissions because electrons emitted from x-ray excited water molecules travel only nanometer distances through matter. The electrons arriving at the gold electrode surface can be detected as an electrical current traveling through a wire attached to it. This avoids confusion with signals from the interior electrolyte because electrons emitted from interior molecules don’t travel far enough to be detected.
There’s an additional problem that arises when studying liquids in contact with working electrodes because they carry a steady current as in batteries and other electrochemical systems. While the emitted electrons from nearby molecules are indeed detectable, this contribution to the current is dwarfed by the normal “Faradaic” current of the battery at finite voltages. When measuring current off the electrode, it is critical to determine which part is due to the x-rays and which is due to the regular battery current.
To overcome this problem, the researchers pulsed the incoming x-rays from the synchrotron at a known frequency. The current contribution resulting from electron emission by interfacial molecules is thus pulsed as well, and instruments can separate this nanoampere modulated current from the main Faradaic current.
These experiments result in absorption vs. x-ray energy curves (spectra) that reflect how water molecules within nanometers of the gold surface absorb the x-rays. To translate that information into molecular structure, a sophisticated theoretical analysis technique is needed.
From spectra to structures
David Prendergast, a staff scientist in the Molecular Foundry and researcher in the Joint Center for Energy Storage Research (JCESR), has developed computational techniques that allow his team to accomplish this translation.
Using supercomputer facilities at Berkeley Lab’s National Energy Research Scientific Computing Center (NERSC), he conducted large molecular dynamics simulations of the gold-water interface and then predicted the x-ray absorption spectra of representative structures from those simulations.
“These are first-principles calculations,” explains Prendergast. “We don’t dictate the chemistry: we just choose what atomic elements are present and how many atoms. That’s it. The chemistry is a result of the calculation.”

Berkeley Lab’s Advanced Light Source is a premier DOE user facility for X-ray spectroscopy. (Photo by Roy Kaltschmidt)
It turns out that for a neutral gold surface, a significant number of water molecules (H2O) next to the gold surface orient with hydrogen (H) atoms pointing toward the gold. Water molecules are bound together by so-called hydrogen bonds, which orient the slightly positively-charged H atoms in each molecule towards the slightly negatively-charged oxygen (O) atoms of neighboring molecules. This network of hydrogen bonds is what holds water molecules together to make a liquid under conditions of temperature and pressure that we consider comfortable as humans. It is perhaps surprising that the inert gold surface can induce significant numbers of water molecules not to hydrogen-bond to each other but to bond to the gold instead. This number is enhanced when the gold is negatively charged and therefore attracting the more positive H atoms. Furthermore, positively-charged gold ions cause water molecules to orient their H atoms away from the gold, which strengthens the hydrogen bond network of the interfacial liquid.
“That’s the main thing we know about the gold electrode surface from the x-ray absorption spectra: how many water molecules are tilted one way or another, and if their hydrogen bonds are broken or not,” concludes Salmeron. “Water next to the electrode has a different molecular structure than it would in the absence of the electrode.”
Exceptionally sensitive
There are a couple of subtle things that are very important, notes Prendergast. First, the shape of the absorption spectra changes as a function of changing voltage. Since the measured spectra agree with the calculations one can draw conclusions about the molecular structure of the liquid interface as a function of voltage. The second is that in the calculations, the change in the structure of water is limited to the first two molecular layers above the surface and these two layers span only about 1 nanometer. To observe any difference in the experimental spectra with varying voltage means that measurements are sensitive to a shorter length scale than was thought possible.
“We had thought the sensitivity to be tens of nanometers, but it turns out to be subnanometer,” says Prendergast. “That’s spectacular!”
This study, which is reported in Science in a paper titled “The structure of interfacial water on gold electrodes studied by x-ray absorption spectroscopy,” marks the first time that the scientific community has shown such high sensitivity in an in-situ environment under working electrode conditions.
The complete list of authors on the paper include Juan-Jesus Velasco-Velez, Chenghao Wu, Tod A. Pascal, Liwen F. Wan, Jinghua Guo, David Prendergast, and Miquel Salmeron.
This research was primarily supported by the DOE Office of Science. The ALS, the Molecular Foundry and NERSC are all DOE Office of Science User Facilities. JCESR is an Energy Innovation Hub funded by DOE’s Office of Science.
ADDITIONAL INFORMATION
For more about the Molecular Foundry go here
For more about the ALS go here
For more about JCESR go here
For more about NERSC go here
For more about the research of Miquel Salmeron go here
For more about the research of David Prendergast go here
# # #
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit the Office of Science website at science.energy.gov/.