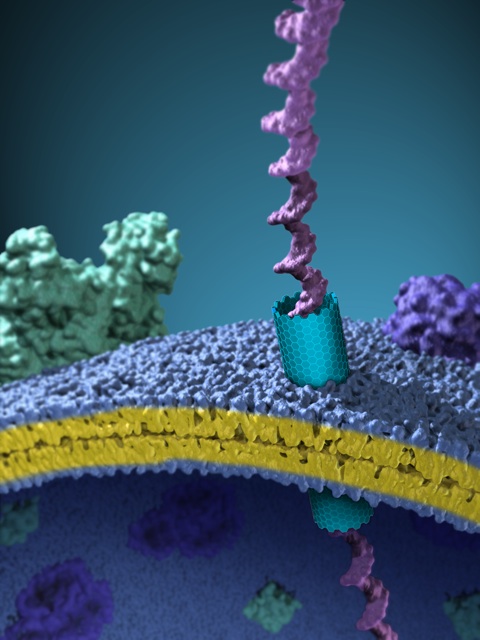
An artist’s interpretation of a nanotube embedded within a cell membrane, allowing a DNA molecule to pass through. Credit: Xavier Studios
Berkeley Lab researchers have helped show that short carbon nanotubes can make excellent artificial pores within cell membranes. Moreover, these nanotubes, which are far more rugged than their biological counterparts, can self-insert into a cell membrane or other lipid bilayers.
“One of the major innovations of this work was figuring out how to make carbon nanotubes the right length and how to get them to insert into a membrane,” says Caroline Ajo-Franklin, staff scientist at the Molecular Foundry at Berkeley Lab.
Ajo-Franklin worked with Alex Noy, a biophysicist at Lawrence Livermore National Laboratory (LLNL) and lead author of the paper describing the research, published in a recent issue of Nature. Colleagues at the University of California Berkeley and the University of Basque Country in Spain also contributed.
To get the nanotubes the right size for a membrane channel, Noy relied on molecules called lipids. Lipids come together to form double-layered barriers, like the lipid bilayer found in cell membranes. When lipids are mixed with longer nanotubes, the lipids assemble around the tubes, indicating the appropriate length for a membrane-bound nanotube. Once the right-sized nanotubes are cut from the longer tube, their lipid coating allows them to infiltrate a cell membrane spontaneously, as the team demonstrated with human kidney cells and Chinese hamster ovary cells.
Luis R. Comolli in the Life Sciences Division at Berkeley Lab (now at the Molecular Biology Consortium) and Frances Allen of the Materials Sciences Division, also at Berkeley Lab, confirmed the short nanotubes successfully implanted themselves within membranes using images from cryogenic transmission electron microscopy.
To test the viability of the nanotubes as transport channels, Noy and Ajo-Franklin designed assays that contained salts and water and measured their flow through the nanotubes. Not only were salts and water able to move easily across the membrane, but the researchers also learned that the ends of the nanotube had a useful property: they could open and close much like biological ion channels.
Thanks to a variety of charges along the edge of the nanotube openings, Ajo-Franklin explains, the openings will close or open in response to the changes in the local ion concentration. This extra level of control in an artificial channel could make the nanotube pores useful for future applications including drug delivery systems or biosensors in which controlling when and how a cell’s membrane is permeable is important.
Additionally, the longevity of nanotubes, which don’t break down as easily as protein-based channels over time or when exposed to heat, make them particularly well-suited for DNA sequencing, a process that relies on these kinds of channels for discerning different base pairs with DNA segments.
This research was supported by the DOE Office of Science and the LDRD program at LLNL.
For more information: Lawrence Livermore National Lab news release here.
For more information about the Molecular Foundry go here.