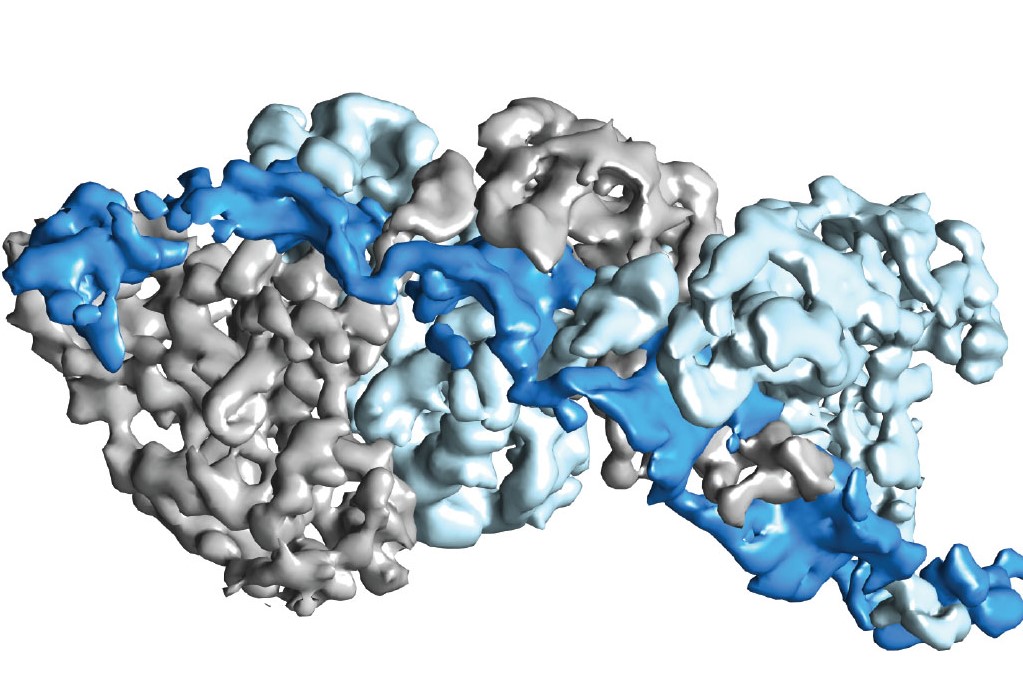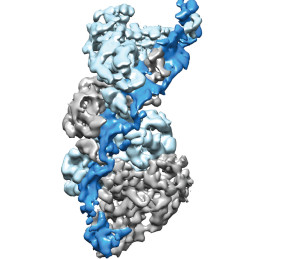
Type III CRISPR-Cas complexes were shown to use a thumb-like domain (top right corner) to target specific sites on an RNA molecule for the destruction of invasive nucleic acids.
A great deal of public attention in the past couple of years has been showered on complexes of bacterial proteins known as “CRISPR-Cas” for their potential use as a tool for editing DNA. Now, researchers with the Lawrence Berkeley National Laboratory (Berkeley Lab) are reporting that CRISPR-Cas complexes could also serve as an engineering tool for RNA, the molecule that translates DNA’s genetic instructions into the production of proteins.
Biochemist Jennifer Doudna and biophysicist Eva Nogales led a study that showed how a CRISPR-Cas surveillance complex in the bacterial immune system is able to target specific sites on an RNA molecule for the destruction of invaders. This targeting mechanism could be adapted by scientists for the genetic engineering of microbes to serve a plethora of purposes, including environmental clean-up, green chemistry, and the production of safer, more effective therapeutic drugs.
“We have provided the first high-resolution structural images of a fully intact Type III CRISPR-Cas surveillance complex and the first picture of how the RNA-targeting mechanism of this complex might work,” says Nogales, a leading authority on electron microscopy.
“Our structural data suggests ways in which this RNA-targeting CRISPR-Cas complex could potentially be re-purposed for RNA-interference applications,” says Doudna, a pioneer in the study and understanding of CRISPR-Cas complexes.
Both Doudna and Nogales hold joint appointments with Berkeley Lab, the University of California (UC) Berkeley, and the Howard Hughes Medical Institute (HHMI). They are the corresponding authors of a paper in Science that describes this research in detail. The paper is titled “Structures of the CRISPR-Cmr complex reveal mode of RNA target positioning.” The lead author is David Taylor, a Damon Runyon Fellow and member of both the Doudna and Nogales research groups. Other co-authors are Yifan Zhu, Raymond Staals, Jack Kornfeld, Akeo Shinkai and John van der Oost
While we humans view bacteria as invading pathogens and have evolved an immune system for protection, bacteria themselves must confront a relentless tide of deadly invaders that include viruses and snippets of nucleic acid known as plasmids. To protect themselves, bacteria have evolved their own immune system. This system is adaptive and nucleic acid-based. It revolves around complexes featuring a genetic element known as CRISPR, which stands for Clustered Regularly Interspaced Short Palindromic Repeats, and an array of CRISPR-associated proteins collectively known as “Cas.” Utilizing small customized CRISPR-derived RNA molecules (crRNA), CRISPR-Cas complexes identify and destroy the DNA of invading viruses and plasmids. There are three distinct types of CRISPR/Cas complexes. In this latest study, Doudna, Nogales and their colleagues focused on the Type III complexes, which recognize and cleave single-stranded RNA.
“Using near-atomic resolution cryo-electron microscopy reconstructions, we learned that Type-III CRISPR-Cas surveillance complexes employ a thumb-like domain intercalation between segments of targeted RNA to orient the RNA for productive cleavage,” Taylor says. “Bacteria use this mechanism to destroy invasive nucleic acids from phages.”
The thumb-like domains used by the Type-III CRISPR-Cas surveillance complexes share a “remarkable” architectural similarity to the thumb-like domains used by Type I CRISPR-Cas complexes for DNA targeting.
“This suggests a divergent evolution of these adaptive immune systems from a common ancestor,” Taylor says. “Future structural and functional studies will be required to determine the atomic details of RNA cleavage that results from the Type-III CRISPR-Cas targeting system.”
This research was primarily supported by the National Institutes of Health.
Additional Information
For more about the research of Jennifer Doudna go here
For more about the research of Eva Nogales go here
# # #
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.