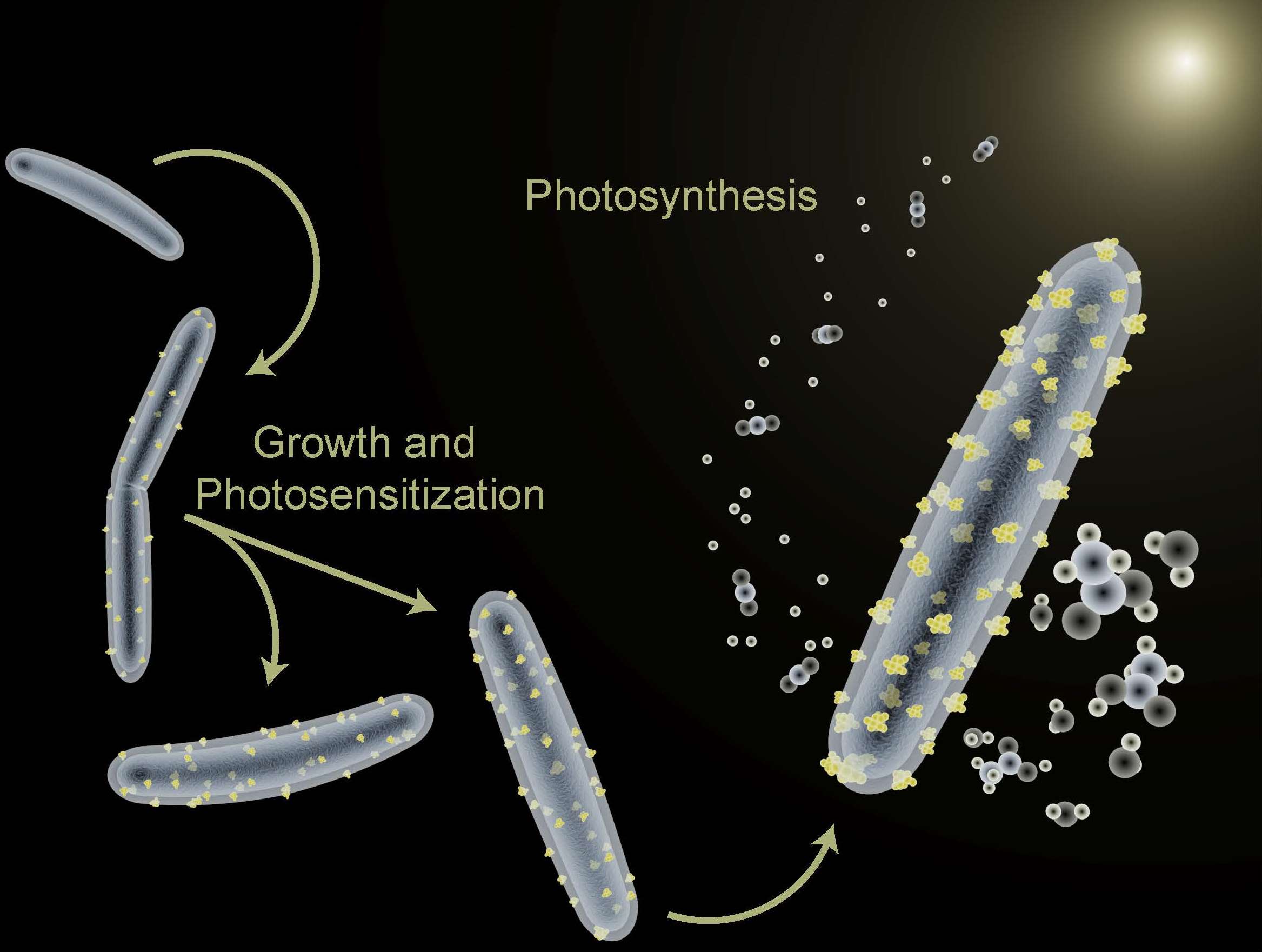
In a 2016 study, Berkeley Lab scientists used the bacterium Moorella thermoacetica in a hybrid artificial photosynthesis system for converting sunlight into valuable chemical products. (Credit: Berkeley Lab)
Several fields of research have sprung up around the chemical drivers, called catalysts, at work in many industrial processes – including those that boost the production of fuels, fertilizers, and foods. These research efforts have developed more useful catalysts that accelerate chemical reactions and make the reactions more efficient without being consumed in the process.
Now, there is growing interest in coordinating the research efforts in these fields to create new, hybrid catalysts with enhanced performance, said Gabor Somorjai, a faculty senior scientist in the Materials Sciences Division at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and chemistry professor at UC Berkeley.
Somorjai, who has authored more than 1,000 journal articles related to catalysis and particularly chemistry occurring at the surface of metals, makes the case for pursuing new forms of hybrid catalysis in a perspective article published this month in the journal Nature Catalysis. Somorjai also has written four textbooks related to surface chemistry.
“Let’s try to combine the results from these fields in new ways,” Somorjai said. “It seems obvious that this will be the evolution of the field.” The paper recognizes some previously published studies relating to hybrid catalysts, and calls for more progress.
Rong “Rocky” Ye, the paper’s lead author, said, “Our paper puts forward the concept of hybrid catalysts systematically and comprehensively so that more researchers will be aware of the possibilities in research directions and collaborations.” Ye is a former graduate student at Berkeley Lab and UC Berkeley who is now a Presidential Postdoctoral Fellow at Cornell University.
The three general types of catalysis research, according to the perspective article, are often categorized as follows:
- Homogeneous catalysts, which typically operate in the same phase of matter (most often a liquid or a gas) as the reactant. A reactant is a substance that participates in a chemical reaction and is changed by the reaction.
- Heterogeneous catalysts, which operate in a different phase of matter from reactants. This type of catalyst can be at the outer or inner surface of dense or porous solids, the article notes, or can be attached to the surface of materials, in the form of metal crystals or nanoparticles, for example.
- Enzyme catalysts, which are 3-D biological molecules derived from amino acids. Enzymes typically are immersed in a liquid.
Creating hybrid catalysts by blending different types of catalysts and reactants “can be greater than the sum of their parts … and may provide a promising method to synthesize elusive end products,” the article states. The perspective, authored by Somorjai and four other Berkeley Lab and UC Berkeley researchers, lists 17 chemical processes that can be sped up by catalysts in each of the above categories.
“When we try to hybridize, we may be able to accelerate the reaction rate or the selectivity for new products,” Somorjai said.
This could open up new avenues for developing longer-lasting batteries and lower-cost medicines, he said.
Already, Somorjai’s research group has found ways to “heterogenize homogenous catalysis” by tethering, grafting, or entrapping catalysts that are typically dissolved in a liquid. This can provide more long-term stability for the reaction processes, reduce waste, and improve the reuse of the catalysts, the perspective notes.
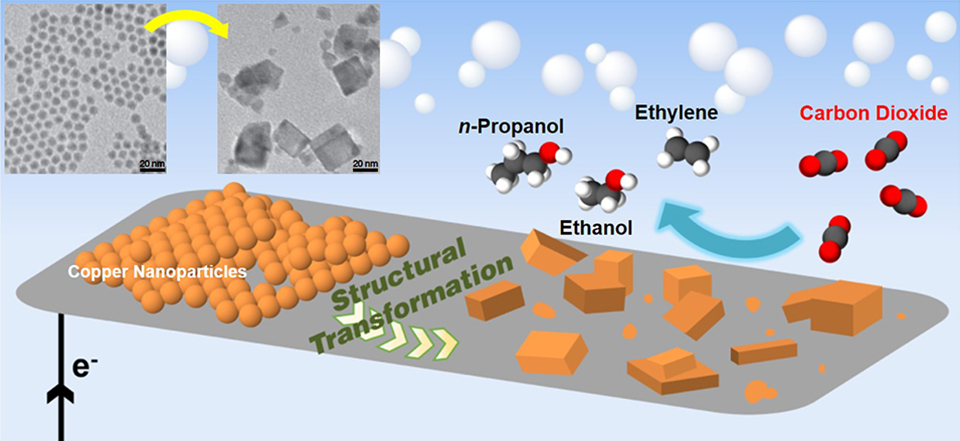
Schematic of a heterogeneous catalyst made of copper nanoparticles that converts carbon dioxide to multicarbon products (ethylene, ethanol, and propanol). Berkeley Lab and UC Berkeley researchers are pursuing designs for hybrid catalysts that can further improve chemical reactions for a range of applications. (Credit: Dohyung Kim/Berkeley Lab)
There are challenges, though, in preventing metals from leaching over time for example, and in optimizing all of the components and conditions to guarantee the superior performance of such hybrids.
Somorjai’s team is now in the early stages of working on hybrid approaches that incorporate enzyme catalysts.
“Let’s try combining them and see what we get – maybe it will be something unique,” Somorjai said. “It seems obvious that this will be the evolution of the field of catalysis.”
Other contributing authors are Jie Zhao, a postdoctoral researcher at UC Berkeley; Brent B. Wickemeyer, an undergraduate researcher at UC Berkeley; and F. Dean Toste, a senior faculty scientist in the Chemical Science Division at Berkeley Lab and chemistry professor at UC Berkeley.
The group’s catalysis work is supported by the DOE Office of Science and the UC Berkeley Student Mentoring and Research Teams program.
More info:
- View the perspective article in Nature Catalysis
- Somorjai Research Group
- “Molecular catalysis science: Perspective on unifying the fields of catalysis,” Proceedings of the National Academy of Sciences, May 10, 2016
- “Integration of the Three Fields of Catalysis: Heterogeneous, Homogeneous, and Enzyme,” 24th International Solvay Conference on Chemistry: Catalysis in Chemistry and Biology.
###
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel Prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.