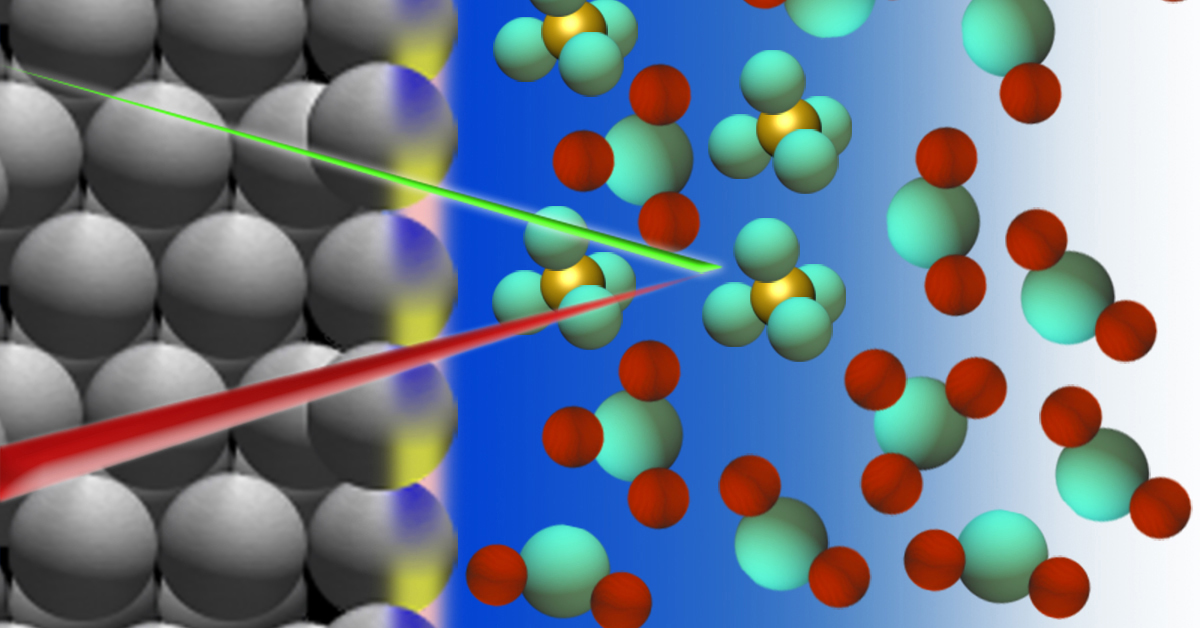
A schematic of the electrochemical cell containing a platinum electrode and sulfuric acid. X-rays are used to study the molecular details at the interface, within only three to four layers of water molecules near the solid surface. (Credit: Lauren Chong/Berkeley Lab)
Understanding the interfaces where solids and liquids meet is key to controlling a wide range of energy-relevant processes, from how batteries store energy to how metals corrode, and more. However, there are many unanswered questions around how these processes work at the atomic or molecular scale.
Now researchers at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) have explored such interfaces and found what they describe as a treasure trove of unexpected results that expands our understanding of working interfaces and how to probe them.
They deployed a powerful X-ray technique to detect the hidden “fingerprints” of various chemical species that collect just above the surface of a platinum electrode immersed in sulfuric acid. They then used supercomputer simulations to make sense of these measurements. This first-of-its-kind study of the molecular structure of the platinum-sulfuric acid interface was recently published in the Journal of the American Chemical Society.
This chemical system – platinum electrodes in a water-based solution of sulfuric acid – is commonly used in chemistry teaching labs to demonstrate the process of splitting water (H2O) into its component elements – hydrogen and oxygen (both gases) – through a process called electrolysis. An external electrical power source, such as a battery, is used to drive electrical charges to the interface between the platinum and the liquid solution and start chemical reactions.
Just before oxygen should be produced, it had long been believed that the surface of the metal electrode starts to corrode or oxidize. What the Berkeley Lab team found challenges the conventional understanding of this electrochemical interface. They found no evidence for the presence of platinum oxide at this stage of the reaction. Instead, the team’s measurements were interpreted as indicating elevated concentrations of sulfate ions near the platinum surface – concentrations that are much higher than those found in the liquid far from the electrode.
“We were very surprised by these results, because it goes against all textbook assumptions,” said study co-author David Prendergast, adding that “the results of this study highlight the importance of multidisciplinary efforts to understand electrochemical processes. Even in apparently well-understood systems, we’ve now shown that there are areas for improvement.”
The team was led by Miquel Salmeron, a senior scientist in Berkeley Lab’s Materials Sciences Division and lead principal investigator of the DOE BES-MSE program Structure and Dynamics of Materials Interfaces, collaborating with Prendergast, a senior staff scientist at Berkeley Lab’s Molecular Foundry, a DOE Office of Science user facility for nanoscience research.
The X-ray spectroscopy technique to probe molecular-scale activities and structure at the electrode surface used X-rays produced at Berkeley Lab’s Advanced Light Source (ALS), also a DOE Office of Science user facility. The technique, developed by Salmeron in 2014, allowed researchers to see molecular details near the solid surface within only three to four layers of water molecules – a distance of at most two nanometers.
Prendergast’s team used theoretical approaches developed at the Molecular Foundry and performed simulations on supercomputers at the National Energy Research Scientific Computing Center (NERSC) at Berkeley Lab to interpret the measurements made at the ALS.
The findings will have a direct impact in scientists’ ability to understand wetting, corrosion, membranes, and electrochemical phenomena. Now that the Berkeley Lab researchers have proven that rust isn’t always a foregone conclusion, they hope to further their work by using X-ray spectroscopy to study how corrosion occurs in copper or iron.
The research was supported by the DOE Office of Science.
# # #
Founded in 1931 on the belief that the biggest scientific challenges are best addressed by teams, Lawrence Berkeley National Laboratory and its scientists have been recognized with 13 Nobel Prizes. Today, Berkeley Lab researchers develop sustainable energy and environmental solutions, create useful new materials, advance the frontiers of computing, and probe the mysteries of life, matter, and the universe. Scientists from around the world rely on the Lab’s facilities for their own discovery science. Berkeley Lab is a multiprogram national laboratory, managed by the University of California for the U.S. Department of Energy’s Office of Science.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
Media contact
Laurel Kellner, [email protected], (510) 590-8034