Scientists discover a surprising first step in the chemical transformation of carbon dioxide using a silver catalyst. (Credit: Berkeley Lab)
Fossil fuels are the lifeblood of modern societies, but their increased use releases carbon dioxide, a climate-warming greenhouse gas, faster than plants can recycle it via photosynthesis.
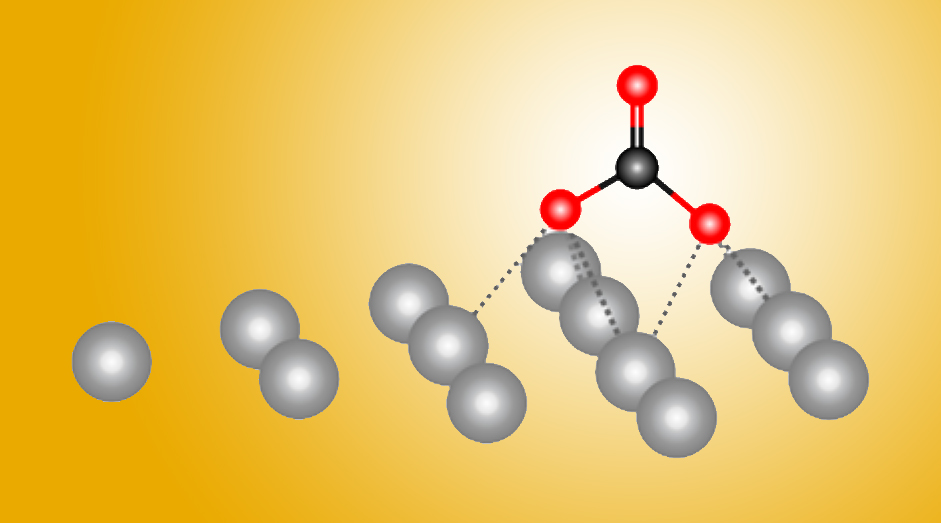
Now, a powerful combination of experiment and theory has revealed atomic-level details about how silver helps transform carbon dioxide gas into a reusable form. The results, reported in the journal Nature Communications, will help in the design of more efficient metal catalysts.
“Before, people always thought that the process was the same on all metals,” said Berkeley Lab researcher Yifan Ye, one of the study’s authors. “But now, we have discovered that there are other options for reactions. This is new chemistry, and it’s a new reaction pathway.”
Metals such as silver facilitate the transformation (or “reduction”) of carbon dioxide into carbon monoxide (CO), which is used to synthesize other useful chemicals. The work revealed a surprising first step in this process that hadn’t been seen nor suggested before. Ultimately, the researchers hope to optimize carbon dioxide catalysis by using additives or metal alloys.
The work involved a close collaboration between theorists from Caltech and experimentalists from Berkeley Lab’s Advanced Light Source, working together under the umbrella of the Joint Center for Artificial Photosynthesis, a Department of Energy Energy Innovation Hub.
Read the full story here.
Media contact:
Laurel Kellner, [email protected], 510-590-8034