
A new technique developed by researchers at Berkeley Lab, in collaboration with Dow and Eindhoven University of Technology in the Netherlands, is providing atomic-resolution details about magnesium chloride, a material involved in the production of the most common plastic, polyethylene – and could help to create a path toward sustainable plastics. (Credit: iStock/Irina Vodneva)
Plastics are all around us – they make up our water bottles, trash bags, packing materials, toys, containers, and more. About 300 million tons of plastic are produced worldwide each year, yet the details of what goes on at the atomic scale during the plastics production process is still unclear.
Now, a new technique developed by researchers at DOE’s Lawrence Berkeley National Laboratory (Berkeley Lab), in collaboration with Dow and Eindhoven University of Technology in the Netherlands, is providing atomic-resolution details about magnesium chloride, a material involved in the production of the most common plastic, polyethylene – and could help to create a path toward sustainable plastics. Their findings were reported in Advanced Functional Materials.
The researchers used pulsed electron beams in an electron microscope to produce first-of-their-kind images of magnesium chloride. A continuous electron beam rapidly damages this delicate, beam-sensitive material, but the new technique allowed the researchers to study it without harm.
“If you had asked me 10 years ago if we could use pulsed electron beams to image beam-sensitive materials with atomic resolution, I would not have believed it,” said Christian Kisielowski, lead author of the study and staff scientist at Berkeley Lab’s Molecular Foundry, a nanoscale science user facility. “Now it is possible, and it has allowed us to study an important material for the plastics industry.”
Kisielowski added that this is a game changer for imaging a wide range of materials that are normally damaged inside an electron microscope. Besides magnesium chloride, for example, pulsed electron beams could also be used to study soft membranes and plastics in general.
Focusing on a new path toward sustainable plastics
Although magnesium chloride is widely used as a support structure for catalysts (materials that speed up reactions) used to make plastics, the exact way in which it works remains a mystery. Atomic-scale images of magnesium chloride would help clarify its role in plastics production and could help pave the way to more specialized and sustainable plastics.
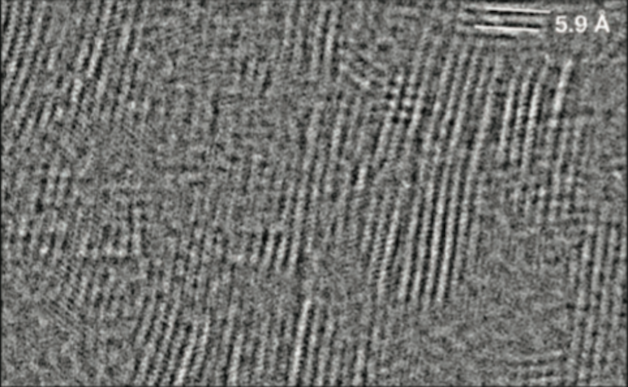
By pulsing the electron beam so that magnesium chloride could “heal” itself between pulses, the researchers preserved the material’s original atomic structure and revealed that sheets of magnesium chloride stack on top of each other in an irregular arrangement. (Credit: Christian Kisielowski/Berkeley Lab)
Unfortunately, previous attempts at imaging this critical material have been difficult because magnesium chloride can exist in two types of crystal structures that have slightly different arrangements of atoms. “The electron beam itself affects the material structure, making it difficult to interpret which structure is being imaged,” said Kisielowski. “By working with our collaborators, we were able to tease out different interactions.”
The Berkeley Lab team collaborated with Eindhoven University of Technology and Dow to develop a technique that delivers periodic pulses of electrons instead of a continuous electron beam to image magnesium chloride. Using a modified electron microscope at Eindhoven, the researchers found that by pulsing the electron beam like an extremely fast strobe light with one pulse every 160 picoseconds (1 picosecond is one trillionth of a second), the material can essentially “heal” itself between pulses.
It’s been well understood that samples get damaged in an electron microscope when atoms are knocked out of position or molecules are split into smaller particles. Through this study, the researchers learned that the accumulation of atomic vibrations caused by the electron beam is equally important. By pulsing the beam in time with these vibrations, the researchers preserved the material’s original atomic structure and revealed that sheets of magnesium chloride stack on top of each other in an irregular arrangement like a haphazard stack of books, which distinguishes it from other materials.
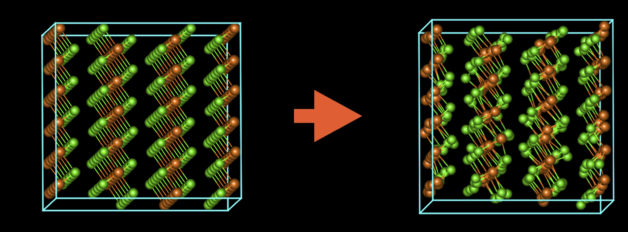
A schematic showing how the arrangement of atoms in magnesium chloride changes as a result of damage from the electron beam. (Credit: Christian Kisielowski/Berkeley Lab)
Another problem that other researchers have grappled with when imaging magnesium chloride is that when the material is exposed to air, it changes in both chemical content and crystal structure (the way its atoms are arranged in space). But when using conventional electron microscopy techniques, the sample is exposed to air as it is transferred to the microscope.
When new solutions become crystal clear
Kisielowski noted that through their collaboration with Dow, they were able to minimize the material’s exposure to air prior to putting it inside the microscope by using a special vacuum-sealed holder. “Our colleagues at Dow taught us how to handle air-sensitive materials, and that was a key element of this whole thing,” Kisielowski said. “We are experts in controlling the electron beam, which is equally important. It was a give-and-take collaboration.”
“Historically, an atomic level understanding of magnesium chloride has been difficult to achieve,” said David Yancey, the project’s Dow collaborator, adding that Dow’s close relationship with Berkeley Lab allowed them to apply the Foundry’s microscopy expertise to solve this challenging issue.
By partnering together, researchers at Berkeley Lab and Dow can tackle fundamental scientific questions that are at the root of challenging industrial problems. “The institutional partnership is opening new avenues for future research,” said Horst Simon, Berkeley Lab’s Deputy Director for Research. “Addressing these big, fundamental questions will lead to far-reaching benefits across science, industry, and the nation’s economy.”
Now that the researchers can image the catalysts for plastics production at atomic resolution, they will move toward studying the relationships between these structures and the properties of plastics, paving the way toward more specialized and sustainable plastics.
“We already know that we have to change how we deal with plastics in the world,” said Petra Specht, second author of the study and a research scientist in the Materials Science and Engineering department at UC Berkeley. “If you want to make changes, you need to know how the process works. Hopefully, our new technique will help us in having a better understanding of how plastics form, and how we can make more sustainable materials,” she added.
The Molecular Foundry is a DOE Office of Science User Facility. This work was supported by the DOE Office of Science, and by a DOE Cooperative Research and Development Agreement between Lawrence Berkeley National Laboratory and Dow.
# # #
Founded in 1931 on the belief that the biggest scientific challenges are best addressed by teams, Lawrence Berkeley National Laboratory and its scientists have been recognized with 13 Nobel Prizes. Today, Berkeley Lab researchers develop sustainable energy and environmental solutions, create useful new materials, advance the frontiers of computing, and probe the mysteries of life, matter, and the universe. Scientists from around the world rely on the Lab’s facilities for their own discovery science. Berkeley Lab is a multiprogram national laboratory, managed by the University of California for the U.S. Department of Energy’s Office of Science.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit energy.gov/science.
Media contact:
Theresa Duque, [email protected], (510) 495-2418
