Scientists have long sought to mimic the process by which plants make their own fuel using sunlight, carbon dioxide, and water through artificial photosynthesis devices, but how exactly substances called catalysts work to generate renewable fuel remains a mystery.
Now, a PNAS study led by Berkeley Lab – and supported by state-of-the-art materials characterization at the Joint Center for Artificial Photosynthesis, powerful X-ray spectroscopy techniques at the Advanced Light Source, and superfast calculations performed at the National Energy Research Scientific Computing Center – has uncovered new insight into how to better control cobalt oxide, one of the most promising catalysts for artificial photosynthesis.
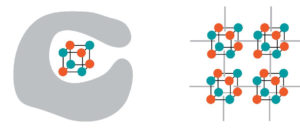
To hold the catalysts in place, the researchers used a MOF as a scaffold (illustration, right) – similar to how tetramanganese, a MOF catalyst in natural photosynthesis, protects itself from self-destruction by hiding in a protein pocket (left). (Credit: Andy Nguyen et al./Berkeley Lab)
When molecules of cobalt oxide cubane, so named for its eight atoms forming a cube, are in solution, the catalytic units eventually collide into one another and react, and thus deactivate.
To hold the catalysts in place, and prevent these collisions, the researchers used a metal-organic framework as a scaffold. The technique is similar to how tetramanganese, a metal-oxygen catalyst in natural photosynthesis, protects itself from self-destruction by hiding in a protein pocket.
“Our study provides a clear, conceptual blueprint for engineering the next generation of energy-converting catalysts,” said Don Tilley, senior faculty scientist in Berkeley Lab’s Chemical Sciences Division and a co-corresponding author of the study.

