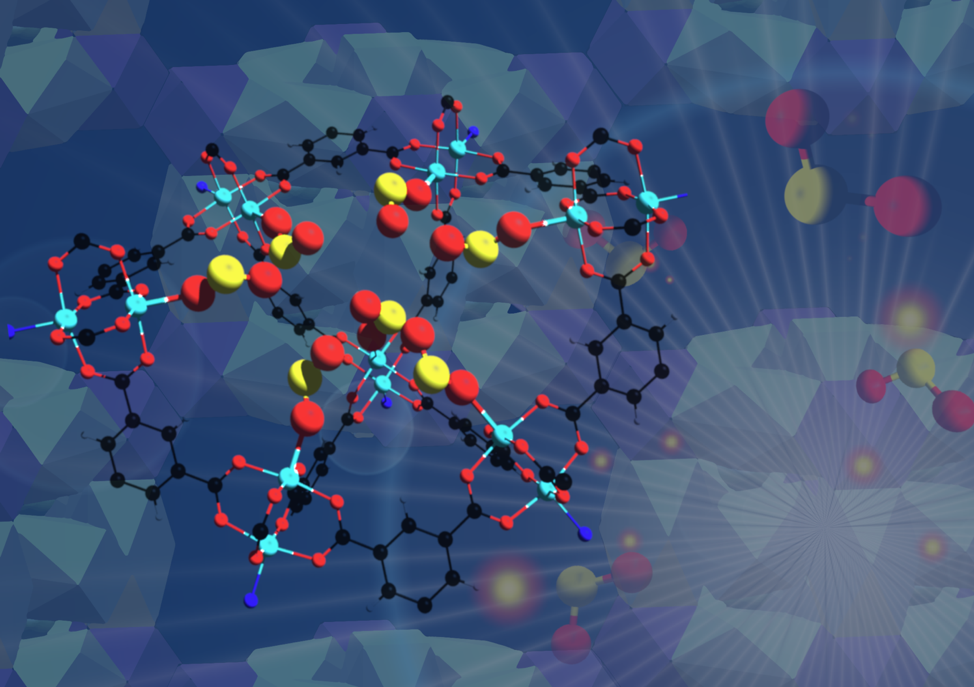
Illustration of sulfur dioxide captured within the MFM-170 material, as revealed by experiments at Berkeley Lab’s Advanced Light Source. (Credit: Gemma Smith/Manchester University)
An international team has developed a robust material that can selectively take in toxic sulfur dioxide gas at record concentrations and preserve it for use in chemical production. The researchers verified its performance using a combination of techniques that included X-ray experiments at Lawrence Berkeley National Laboratory’s (Berkeley Lab’s) Advanced Light Source (ALS).
Sulfur dioxide emissions are typically produced by power plants, other industrial facilities, and trains, ships, and heavy equipment, and can be harmful to human health and the environment. The team developed porous, cagelike, stable copper-containing molecules known as metal-organic frameworks or MOFs that are designed to separate sulfur dioxide (SO2) gas from other gases. The team exposed the MOF material, dubbed MFM-170, to simulated exhaust gases and found that it efficiently separated out SO2 from the gas mixture at elevated temperatures even in the presence of water.
Existing techniques to remove SO2 from pollution streams can produce a lot of solid and liquid waste and may only remove 60-95% of the toxic gas, researchers noted, while the MOF has been shown to eliminate SO2 down to a level below 0.1 parts per million – or 99.99999% SO2-free. Their study was published Oct. 14 in the journal Nature Materials.
The team, led by University of Manchester scientists, used X-rays produced at the ALS to explore the detailed molecular structure of the MOF crystals. They also performed experiments at Oak Ridge National Laboratory; Diamond Light Source, ISIS Neutron and Muon Source, and Manchester University in the U.K.
More info: Sulfur Dioxide: from toxic gas to industrial ingredient