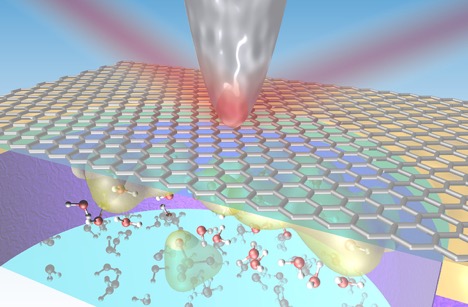
Infrared light is focused onto the sharp metallic tip of an atomic force microscope, enabling the acquisition of vibrational spectra from a graphene-liquid interface. (Credit: Artem Baskin, Jonathon Larson/Berkeley Lab)
How a liquid interacts with the surface of a solid is important in batteries and fuel cells, chemical production, corrosion phenomena, and many biological processes.
To better understand this solid-liquid interface, researchers at Berkeley Lab developed a platform to explore these interactions under real conditions (“in situ”) at the nanoscale using a technique that combines infrared light with an atomic force microscopy (AFM) probe. The results were published in the journal Nano Letters.
The team explored the interaction of graphene with several liquids, including water and a common battery electrolyte fluid. Graphene is an atomically thin form of carbon. Its single-layer atomic structure gives the material some unique properties, including incredible mechanical strength and high electrical conductivity.
Researchers used a beam of infrared light produced at Berkeley Lab’s Advanced Light Source and they focused it at the tip of an AFM probe that scanned across a section of graphene in contact with the liquids. The infrared technique provides a nondestructive way to explore the active nanoscale chemistry of the solid-liquid interface.
By measuring the infrared light scattered from the probe’s tip, researchers collected details about the chemical compounds and the concentration of charged particles along the solid-liquid interface. The same technique, which revealed hidden features at this interface that were not seen using conventional methods, can be used to explore a range of materials and liquids.
Researchers from the Lab’s Materials Sciences Division, Molecular Foundry, and Energy Storage and Distributed Resources Division participated in the study. The Molecular Foundry and Advanced Light Source are DOE Office of Science user facilities.
More info: