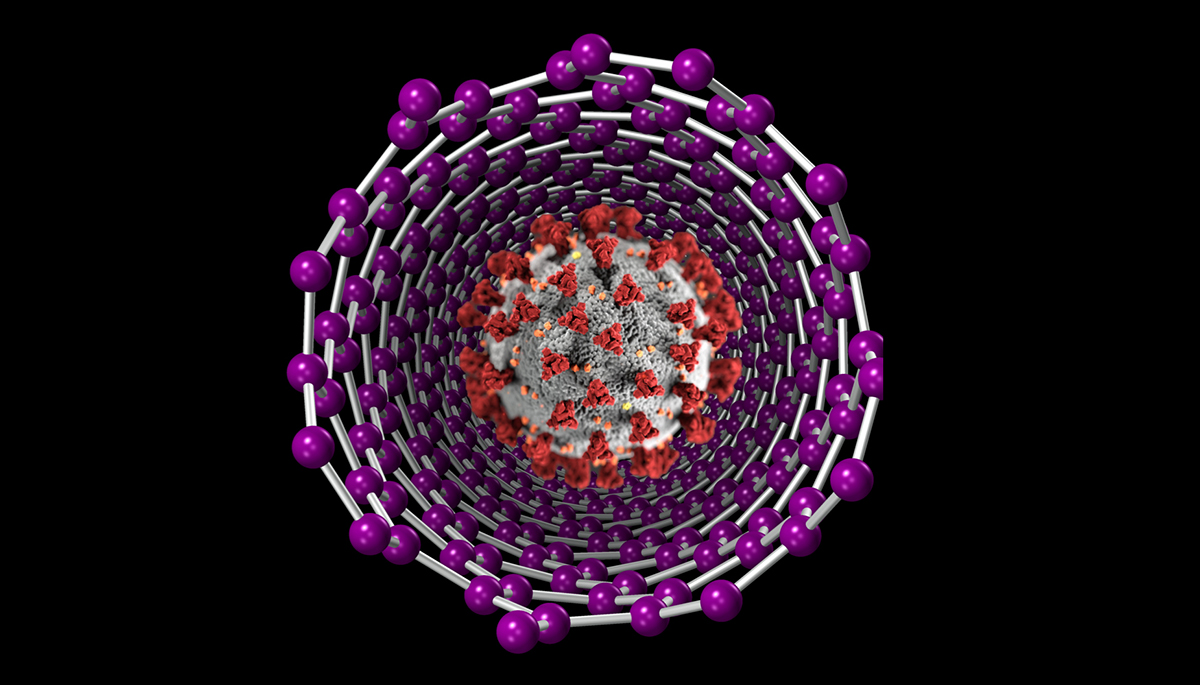
Artist’s rendition of a carbon nanotube COVID-19 detector. (Courtesy of: Zettl Research Group/Berkeley Lab)
A technology spun from carbon nanotube sensors discovered 20 years ago by Lawrence Berkeley National Laboratory (Berkeley Lab) scientists could one day help health care providers test patients for COVID-19, the disease caused by the coronavirus SARS-CoV-2.
When Alex Zettl, Marvin Cohen, and their research teams at Berkeley Lab first demonstrated ultrasensitive oxygen sensors devised from carbon nanotubes – hollow carbon wires with walls no thicker than an atom – they envisioned a broad spectrum of applications, such as gas-leak detectors or air- and water-pollution detectors.
Subsequent studies out of Zettl’s lab revealed that carbon nanotubes – or CNTs – could also be used to detect proteins or carbohydrates at the level of single cells for biological and medical applications. “CNTs’ exquisite chemical sensitivity had dramatic life-science implications that could benefit society,” Zettl said.
But since Zettl normally investigates atomically thin materials known as nanomaterials for the Department of Energy’s “Novel sp2-Bonded Materials and Related Nanostructures” program, his lab is set up for launching exciting new experiments in quantum physics, not new applications for entrepreneurial startups.
So in 2000, Zettl and Cohen, who are both senior faculty scientists in Berkeley Lab’s Materials Sciences Division and physics professors at UC Berkeley, branched out into the world of commercial spinoffs by co-founding the Emeryville-based biotech company Nanomix Inc. They currently sit on the company’s board of directors – Zettl participates as an adviser, and Cohen as a member.
Today, the company is one of many U.S. companies vying for FDA Emergency Use Authorization (EUA) to deploy new diagnostic tests for COVID-19.
Last month, the company was awarded approximately $570,000 in funding from the Department of Health and Human Services’ Biomedical Advanced Research and Development Authority (BARDA) to develop disposable cartridges that test for protein traces of the coronavirus – known as antigens – in nasal swab samples, and for antibodies to the coronavirus in blood samples.
Patient samples loaded onto the cartridges are analyzed by the Nanomix “eLab,” a handheld testing device the company first developed more than five years ago in response to the Ebola virus epidemic.
The cartridges rely on tiny carbon biosensors modeled after the Zettl and Cohen labs’ groundbreaking carbon nanotube technology to detect coronavirus antigens during the early stages of a current infection. In addition, the cartridges can test for antibodies the immune system builds up as part of our body’s natural defense mechanism against a previous SARS-CoV-2 infection. The company says the eLab system can produce test results in about 15 minutes.
If granted FDA Emergency Use Authorization, the company hopes to have COVID-19-ready eLab products available for health care providers in June, and to scale up its supply and production capacity to provide hundreds of thousands of test kits, said Nanomix President and CEO David Ludvigson.
“The fact that my research could help so many people is very rewarding. I’m happy that I was able to contribute in that way,” Zettl said.
For more information:
- “Nanotubes: Surprising Sensitivity to Oxygen Opens New Possibilities,” Berkeley Lab news release, March 29, 2000
- “Carbon Nanotubes You Can Live With,” Berkeley Lab news release, July 26, 2006