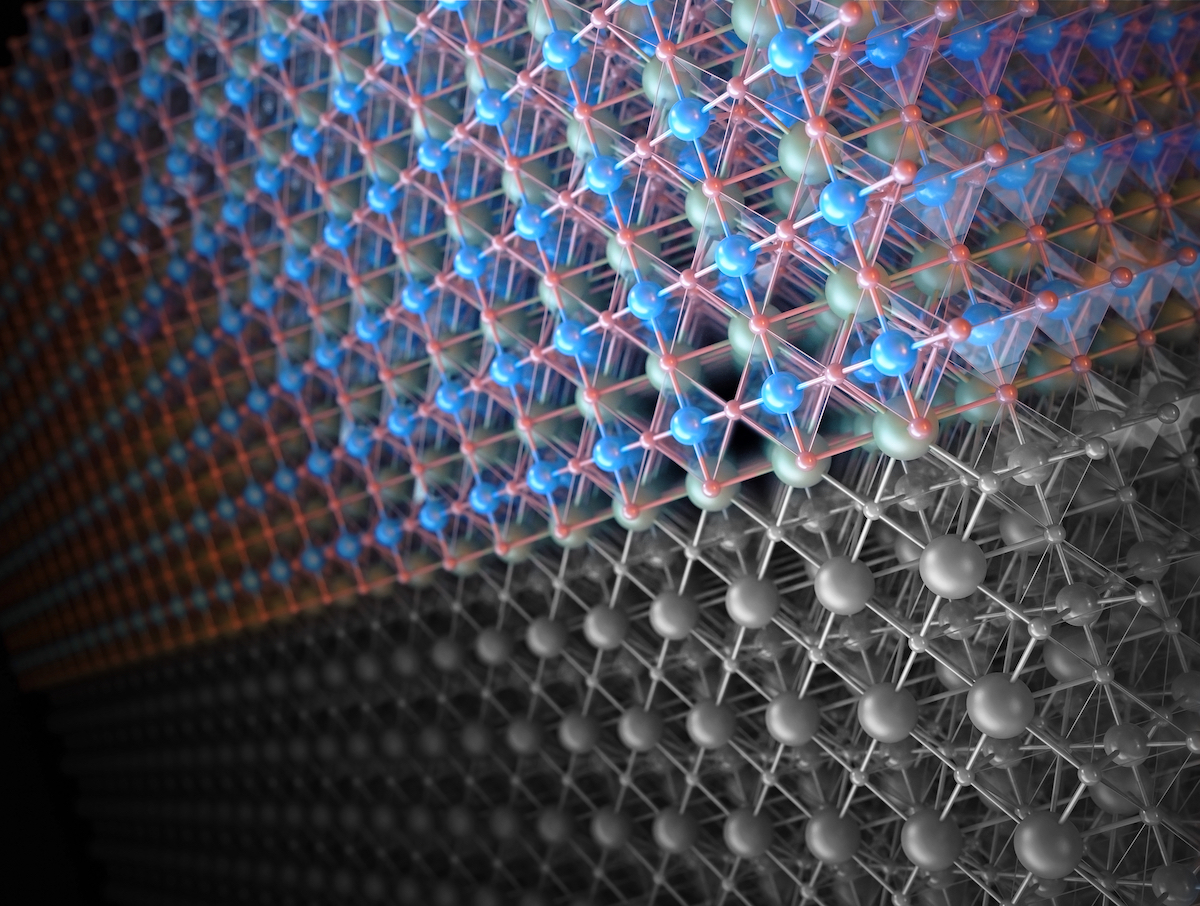
This illustration shows two possible types of surface layers for a catalyst that performs the water-splitting reaction, the first step in making hydrogen fuel: The gray surface is lanthanum oxide and the colorful surface is nickel oxide. A rearrangement of nickel oxide’s atoms while carrying out the reaction made it twice as efficient. Researchers hope to harness this phenomenon to make better catalysts. Lanthanum atoms are depicted in green, nickel atoms in blue, and oxygen atoms in red. (Credit: CUBE3D)
X-ray experiments at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) revealed an unexpected transformation in a single atomic layer of a material that contributed to a doubling in the speed of a chemical reaction – the splitting of water into hydrogen and oxygen gases. This process is a first step in producing hydrogen fuel for applications such as electric vehicles powered by hydrogen fuel cells.
The research team, led by scientists at SLAC National Accelerator Laboratory, performed a unique X-ray technique and related analyses, pioneered at Berkeley Lab’s Advanced Light Source (ALS), to home in on the changes at the surface layer of the material. The ALS produces X-rays and other forms of intense light to carry out simultaneous experiments at dozens of beamlines.
“There is simply no other place in the world that can do these analyses on the level that the ALS can right now,” said Slavomír Nemšák, a beamline scientist at the ALS who contributed to the study, published Jan. 11 in Nature Materials.
The technique they used allowed them to probe the surface of a catalyst material called lanthanum nickel oxide (LNO) that is useful in water splitting. Catalysts are used to speed up or otherwise improve the efficiency of chemical reactions.
The catalyst was engineered in precise layers, and was about 100 atoms thick. Samples were prepared with either a nickel-rich or a lanthanum-rich surface. The samples with the nickel-rich layers carried out the water-splitting reaction twice as fast, and the atomic structure had transformed from a cubic to hexagonal pattern in the last atomic layer.
“The ALS helped to reveal this difference,” Nemšák said. “This technique brought extremely precise depth-specific information on the chemical composition of the catalysts.” Computer simulations performed at Berkeley Lab’s National Energy Research Scientific Computing Center (NERSC) confirmed the catalyst’s hexagonal structure would be more active and stable than the cubic structure.
More
- Read a related SLAC National Accelerator Laboratory press release, “Study Shows Tweaking One Layer of Atoms on a Catalyst’s Surface Can Make It Work Better,” Jan. 11, 2021.