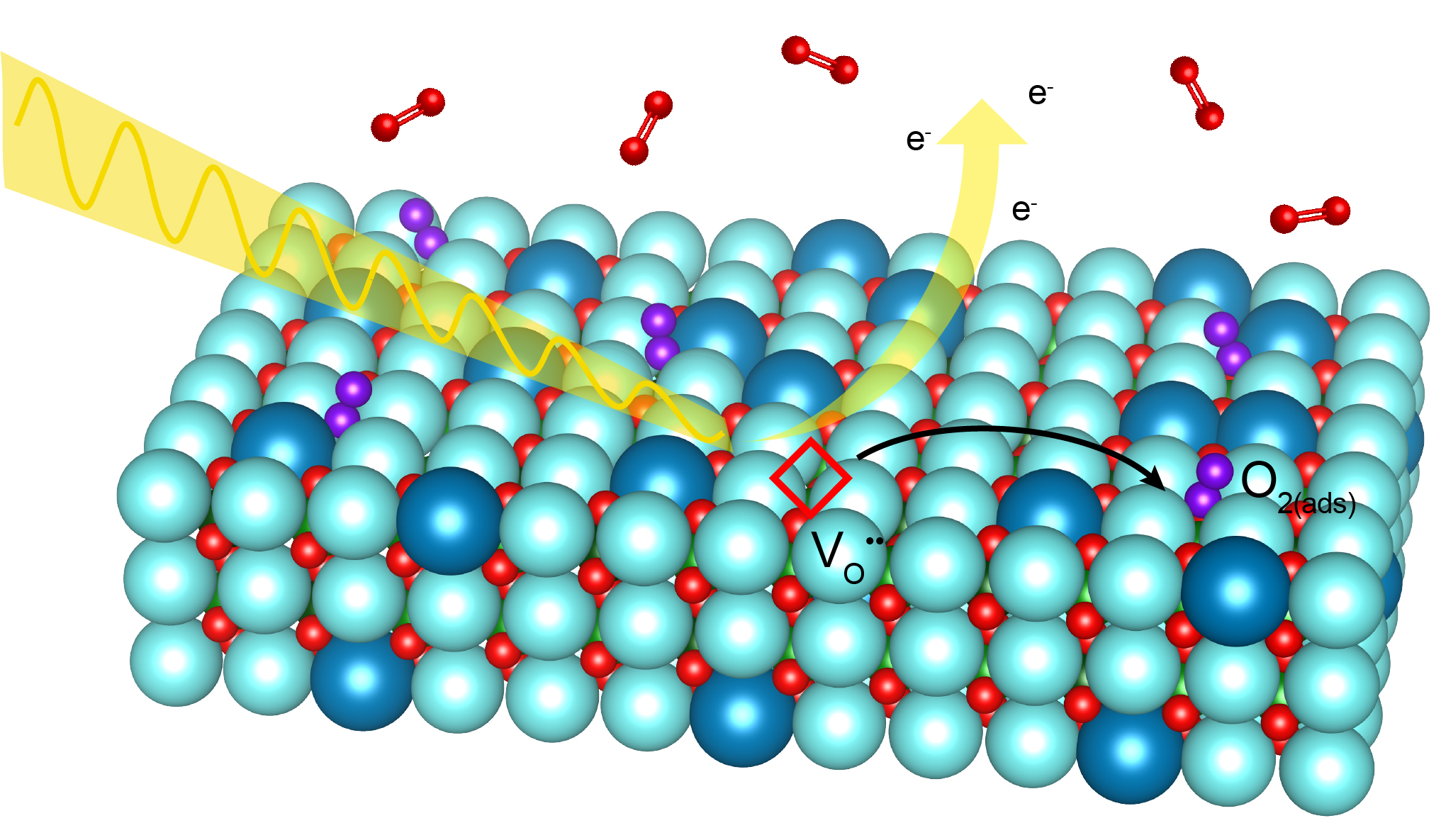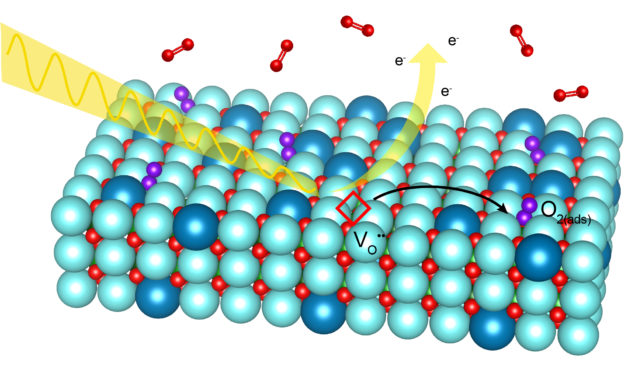
X-ray beams allowed researchers to “see” oxygen gas molecules adhere to a specially prepared electrode surface, an important step in the electrochemical reaction taking place in fuel cells. (Credit: Abel Fernandez/UC Berkeley)
Solid oxide fuel cells (SOFCs) are a promising technology for cleanly converting chemical energy to electrical energy. But their efficiency depends on the rate at which solids and gases interact at the devices’ electrode surfaces. Thus, to explore ways to improve SOFC efficiency, an international team led by researchers from Berkeley Lab studied a model electrode material in a new way – by exposing a different facet of its crystal structure to oxygen gas at operating pressures and temperatures.
 Berkeley Lab’s new Energy Storage Center aims to foster impactful collaborations with national labs, universities, and industry to accelerate innovative research in a number of areas, including materials synthesis, advanced characterization, and battery testing and failure analysis. If you have questions about the Berkeley Lab Energy Storage Center, email [email protected].
Berkeley Lab’s new Energy Storage Center aims to foster impactful collaborations with national labs, universities, and industry to accelerate innovative research in a number of areas, including materials synthesis, advanced characterization, and battery testing and failure analysis. If you have questions about the Berkeley Lab Energy Storage Center, email [email protected].
“We began by asking questions like, could different reaction rates be achieved from the same material, just by changing which surface the oxygen reacts with?” said Lane Martin, a faculty scientist in Berkeley Lab’s Materials Sciences Division. “We wanted to examine how the atomic configuration at specific surfaces of these materials makes a difference when it comes to reacting with the oxygen gas.”
Thin films of a common SOFC cathode material, lanthanum strontium cobalt ferrite (LSCF), were synthesized to expose a surface that was oriented along a diagonal crystallographic plane. Electrochemical measurements on this atypical surface yielded oxygen reaction rates up to three times faster than those measured on the usual horizontal plane.
To better understand the mechanisms underlying this improvement, the researchers used Berkeley Lab’s Advanced Light Source (ALS) to probe the “new” surface at high temperatures and in varying pressures of oxygen. The results revealed that different crystallographic planes stabilize different surface chemistries, even though the chemistry in the bulk of the films is unchanged.
“Exposing different surfaces to air can lead to completely different structures, chemistries, and defect concentrations to a point where these surfaces almost look and act like different materials,” said Abel Fernandez, a graduate student in Materials Science and Engineering at UC Berkeley and co-first author of the study. “Taking our results into consideration can allow manufacturers a relatively simple way to enhance the reactivity of LSCF-based cathodes without the groundwork typically necessary for utilizing new materials chemistries.”
More: