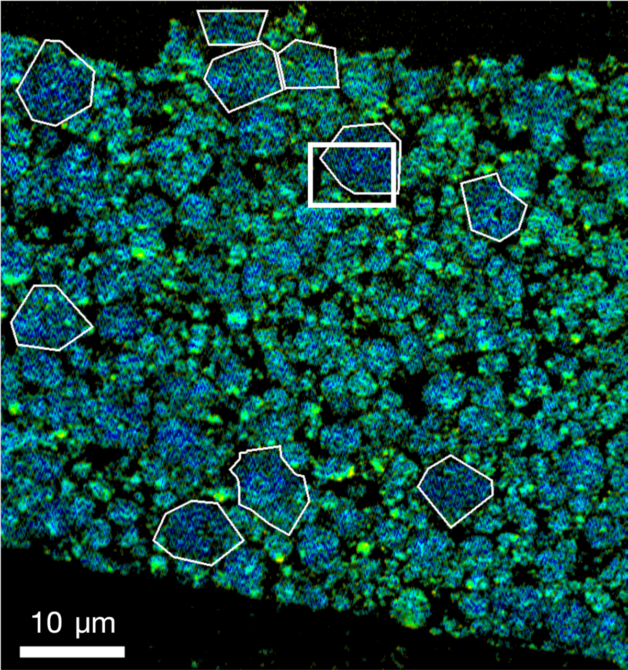
Detailed X-ray measurements at the Advanced Light Source helped a research team co-led by Berkeley Lab, SLAC, and Stanford University reveal how oxygen seeps out of the billions of nanoparticles that make up lithium-ion battery electrodes. (Credit: Berkeley Lab)
Adapted from a SLAC news release by Glennda Chui.
Lithium-ion batteries work like a rocking chair, moving lithium ions back and forth between two electrodes that temporarily store charge. Ideally, those ions are the only things moving in and out of the billions of nanoparticles that make up each electrode.
But researchers have known for some time that oxygen atoms leak out of the particles as lithium moves back and forth. The details have been hard to pin down because the signals from these leaks are too small to measure directly.
Now, in a study published in Nature Energy, a research team co-led by SLAC National Accelerator Laboratory, Stanford University, and Berkeley Lab has measured this process with unprecedented detail, showing how the holes, or vacancies, left by escaping oxygen atoms change the electrode’s structure and chemistry and gradually reduce how much energy it can store.
 Berkeley Lab’s new Energy Storage Center aims to foster impactful collaborations with national labs, universities, and industry to accelerate innovative research in a number of areas, including materials synthesis, advanced characterization, and battery testing and failure analysis. If you have questions about the Berkeley Lab Energy Storage Center, email [email protected].
Berkeley Lab’s new Energy Storage Center aims to foster impactful collaborations with national labs, universities, and industry to accelerate innovative research in a number of areas, including materials synthesis, advanced characterization, and battery testing and failure analysis. If you have questions about the Berkeley Lab Energy Storage Center, email [email protected].
Using COSMIC, a multipurpose X-ray instrument at Berkeley Lab’s Advanced Light Source (ALS), the research team scanned across samples of electrode nanoparticles, making high-res images and probing the chemical makeup of each tiny spot. This information was combined with a computational technique called ptychography to reveal nanoscale details, measured in billionths of a meter.
At SLAC’s Stanford Synchrotron Lightsource, the team shot X-rays through entire electrodes to confirm that what they were seeing at the nanoscale level was also true at a much larger scale.
Comparing the experimental results with computer models of how oxygen loss might occur, the team concluded that an initial burst of oxygen escapes from the surfaces of particles, followed by a very slow trickle from the interior. Where nanoparticles glommed together to form larger clumps, those near the center of the clump lost less oxygen than those near the surface.
The results contradict some of the assumptions scientists had made about this process and could suggest new ways of engineering electrodes to prevent it.
“Previously, researchers were not able to access the length scales needed to study oxygen release in batteries from the primary particle to the electrode level. COSMIC’s ability to achieve few-nanometer spatial resolution with chemical specifity across a wide field allowed us, for the first time, to study the influence of microstructure on this phenomenon,” said co-senior author David Shapiro, who is the lead scientist for COSMIC’s microscopy experiments. Shapiro also leads the ALS Microscopy Program.
