Contact: Lynn Yarris, (510) 486-5375, [email protected]
October of this year marked the 25th anniversary of the National Breast Cancer Awareness Month organization, a partnership of public service groups, medical associations and government agencies that provides informational resources on a disease that remains one of the leading forms of cancer to strike women in the United States. When the NBCAM began its campaign in 1984, the talk in breast cancer research was almost exclusively about oncogenes and genetic mutations. However, two years earlier, a theory had been quietly introduced that would eventually change the conversation in a profound manner. Today the talk in breast cancer, as well as in many other forms of cancer, has broadened beyond genetics to include the microenvironment and other factors. With this expanded conversation has come the very real possibility that cancer may one day be transitioned from a life-threatening to a manageable condition.
Mina Bissell, a Berkeley Lab Distinguished Scientist and multiple award-winning cell and cancer biologist, is now universally recognized as the pioneer who uncovered the critical role in cancer development played by a breast cell’s microenvironment. However, in 1982, when she first proposed that the extracellular matrix (ECM), a network of fibrous and globular proteins immediately surrounding a breast cell, is crucial to the breast cell’s normal functioning, and that ECM loss or damage can lead to malignancy, her theory was not well received. Conventional scientific wisdom at that time held the ECM to be nothing more than an inert scaffold upon which cells grew and developed.

Mina Bissell is a Berkeley Lab Distinguished Scientist and an award-winning cell biologist specializing in breast cancer research. She says the 20th century will be remembered for the discovery of how genetic defects contribute to cancer, but the 21st century will be remembered for the cellular microenvironment that makes up the context of cancer. (Photo Roy Kaltschmidt)
“Very few people wanted to talk about the ECM and the microenvironment at that time because they thought it seemed messy and unimportant,” Bissell says. “Because my training had been in chemistry and molecular genetics, I studied bacteria as a graduate student at Harvard in the late 1960s. Those bacterial studies made me aware of the profound effects that microenvironmental factors can have on the activation and repression of genes.”
Cancerous and healthy cells share much of the same basic biology and biochemistry, but in the cancerous cells, the expression or suppression of genes is improperly regulated. As a result, cancer cells exhibit uncontrolled growth and abnormal differentiation. It was Bissell’s contention that a cancer cell loses the ability to “sense” its microenvironment properly. Working initially with mammary cells from pregnant mice, she and a succession of students and postdoctoral fellows performed a series of experiments over the next two decades in which the microenvironments of cells in cultures were manipulated in various manners. Like a puzzle being assembled piece-by-piece, a clear picture of how the ECM and the rest of a cell’s microenvironment regulate cell function emerged. The results carried tremendous implications for breast cancer therapy.
“All the cells in your body have the same genetic material or genotype and theoretically should all give the same message, but this is not the case,” Bissell told attendees of the Era of Hope breast cancer conference in Philadelphia in 2005. “No cell is an island. All cells are surrounded by their own unique microenvironment including other cells and their ECM. It is the microenvironment that determines a cell’s phenotype – nose, liver, breast, etc., – and phenotype can trump a cell’s genotype.”
The dominance of phenotype over genotype can explain why not every woman who carries a BRCA 1 or BRCA 2 genetic mutation develops cancer in every one of her breast cells, and why some women with a BRCA oncogene never develop cancer at all.
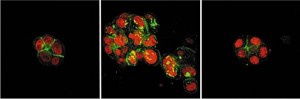
This image shows a cluster of normal breast cells on the left, tumor cells in the center, and cells which have reverted to normal phenotype on the right after signals from the microenvironment were blocked. Areas shown in red are cell nuclei; the green areas are cytoskeletal proteins.
One of the most startling of all the findings of Bissell’s studies was the revelation that manipulating the cellular microenvironment and their signals to the cells can allow malignant cancer cells to “revert” to a normal phenotype. Her studies also revealed that the response of breast cancer cells to a therapeutic agent can be modified by the microenvironment, a finding made possible by Bissell’s unique and extensive use of three-dimensional cultures.
“Cell growth and malignant behavior are regulated at the level of tissue organization and in three dimensions,” she says. “If the tissue structure and the microenvironment are the message, then tumor cells with abnormal genomes should be capable of becoming phenotypically normal if that structure is restored. Furthermore, destruction of the microenvironment and the tissue structure by itself could be a carcinogenic event. We have since proved both of these predictions!”
In response to the ground-breaking research of Bissell and her collaborators, as well as others who have since contributed to this field, the National Cancer Institute (NCI) created review panels to study the role of the tumor microenvironment and in 2006 launched the Tumor Microenvironment Network. The TMN funds research programs aimed at learning more about the role of the tumor microenvironment in cancer initiation, progression and metastases.
Most recently, the National Institutes of Health (NIH) awarded Bissell a prestigious MERIT award, which stands for Method to Extend Research in Time. Bissell received her MERIT award to continue her investigations into what she terms the “dynamic reciprocity” that exists not only between a normal cell and its surroundings, but also between a tumor and its microenvironment.
Yet another indicator of the degree to which Bissell’s research has changed the breast cancer conversation is the emergence of new therapies that specifically target the microenvironment. Though still in the early stages of development, several different strategies for microenvironmental therapies as well as regenerative medicine are now being moved forward.
“These proposed therapies reflect the growing acceptance that the ultimate fate of a cell in a woman’s breast – whether it develops normally or whether it turns cancerous – depends upon microenvironmental signals,” Bissell says. “Therapies that modulate such signals have the potential to normalize malignant cells, or to create conditions under which tumor cells remain dormant.”
At the core of Bissell’s breast cancer research has always been her conviction that to make significant advances in the field of cell biology as a whole, scientists must think outside the cell. When she first proposed her ECM theory in the Journal of Theoretical Biology, she wrote that understanding how the cell microenvironment regulates gene expression will require “the combined efforts of biologists, chemists and perhaps even physicists and engineers since the postulated dynamic reciprocity is undoubtedly physical as well biochemical.”
Those words proved prophetic during this past National Breast Cancer Awareness Month when the NCI announced a $15.7 million, five-year grant designed to bring physicists and engineers into the fight against cancer. Awarded to a partnership that includes Berkley Lab and the University of California’s Berkeley and San Francisco campuses, this grant looks to provide what Bissell called for nearly three decades earlier, a better understanding of the influences outside a cell – in this case physical forces – that help determine the cell’s ultimate fate.
“No single scientific discipline can answer the complexity of the biological problems we confront today,” Bissell says. “Many existing paradigms need to be broken down to understand why we become who we are biologically, how we maintain specificity with a constant genome in 10 trillion cells, why and how we age, and why controls break down as we get sick and/or get cancer.”
Additional Information
For more information about the research of Mina Bissell, visit her Website at
http://www.lbl.gov/lsd/People_&_Organization/Scientific_Staff_Directory/Bissell_Lab.html
Watch a video of Mina Bissell here
http://videoglossary.lbl.gov/2009/cellular-microenvironment/
For more about the National Breast Cancer Awareness Month organization:
For more about the National Cancer Institute’s
Tumor Microenvironment Network: http://tmen.nci.nih.gov/