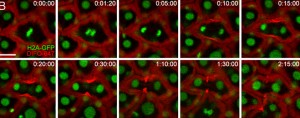
Time-lapse monitoring of zebrafish embryos during cell division after the embryos were microinjected with azido sugars, allowed to develop for 10 hours, then reacted with DIFO. Top right of each image shows elapsed time (h:min:sec). Red marks the DIFO signal around the cell membrane, green identifies cell nuclei. (Image from Bertozzi group)
Researchers with the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and the University of California (UC), Berkeley, have successfully attached imaging probes to glycans – the sugar molecules that are abundant on the surfaces of living cells – in the embryos of zebrafish less than seven hours after fertilization. Glycans are key regulators of the processes that guide cell development, and zebrafish are a top vertebrate model organism of embryogenesis. This new technique enables scientists to study the physiological changes cells undergo during embryogenesis without invading and doing damage to the embryos.
The team of researchers led by Carolyn Bertozzi, a Berkeley Lab-UC Berkeley chemical biologist and leading authority on glycobiology, used a combination of glycan metabolic labeling and copper-free click chemistry to record the earliest images ever of glycan activity on embryonic cells. The images were obtained during a stage of development in which many of the cells were still in the “multipotent stem cell” state, meaning they had yet to differentiate into specific tissue types.
“We know from earlier studies of developmental biology that glycan structures can change a lot during the early stages of embryogenesis,” Bertozzi says. “With this new technology, we hope to witness some of those changes in the glycome in real time and to understand better how cell surface glycans might contribute to the decisions that stem cells make about their destiny.”

A research team led by Carolyn Bertozzi (seated) and including (from left) Scott Laughlin, Sharon Amacher and Karen Dehnert successfully attached imaging probes to glycans in the embryos of zebrafish less than seven hours after fertilization, the earliest recordings ever of of glycan activity on embryonic cells. (Photo by Roy Kaltschmidt, Berkeley Lab Public Affairs).
Bertozzi is the director of Berkeley Lab’s Molecular Foundry, a faculty scientist with Berkeley Lab’s Materials Sciences and Physical Biosciences Divisions, and the T.Z. and Irmgard Chu Distinguished Professor of Chemistry as well as a professor of Molecular and Cell Biology at UC Berkeley, and an investigator with the Howard Hughes Medical Institute (HHMI).
She is also the principal investigator and corresponding author on a paper published in the Proceedings of the National Academy of Science (PNAS) that describes this latest glycan imaging research. The paper is titled “Visualizing enveloping layer glycans during zebrafish early embryogenesis.” Co-authoring the paper with Bertozzi were graduate students Jeremy Baskin, Karen Dehnert and Scott Laughlin, and Sharon Amacher, Associate Professor of Molecular and Cell Biology at UC Berkeley.
Embryogenesis is the process by which a fertilized egg develops into a fetus. It starts with a single zygote cell that multiplies through rapid division (mitosis) into stem cells that subsequently differentiate into the specific types of cells that make up organs and tissues. Biologists need non-invasive imaging techniques to capture in detail such developments at the molecular as well as the cellular levels. Glycans play a central role in the signaling that takes place between cells during embryogenesis, which makes them appealing targets for molecular imaging. However, prior to Bertozzi’s research, glycans were difficult to visualize using the standard tools of molecular imaging.
In 2007, Bertozzi and her research group announced the first copper-free variant of a chemical reaction known as “click chemistry,” one of the most proficient methods for attaching probes to biological molecules. Conventional click chemistry requires a copper catalyst to accelerate the reaction of alkynes with azides – functional groups featuring three nitrogen atoms that can be integrated into biomolecules by metabolic labeling. However, because of copper’s toxicity, the original click chemistry reactions could only be used on fixed cells or cells in a test-tube. Bertozzi and her group extended click chemistry to living cells and organisms by developing a difluorinated cyclooctyne or DIFO reagent that reacts with azides at physiological temperatures, eliminating the need for the toxic catalyst.
“It is a two-step chemical strategy for labeling glycans with imaging agents in vivo,” Bertozzi says. “It entails metabolic labeling with synthetic azido sugars that hijack glycan biosynthesis, followed by covalent chemical tagging of the azide-labeled glycans with a compound bearing both an azide-reactive group, such as DIFO, and an imaging probe.”
Two years ago, Bertozzi and her group used their copper-free click chemistry technique to image cells in live zebrafish embryos, which by virtue of being transparent, are popular for scientists studying embryogenesis. Those studies revealed dramatic differences in cell-surface expression, intracellular trafficking patterns, and tissue distributions of glycans at different stages of zebrafish larval development.
“However, we were unable to detect labeled glycans in zebrafish embryos earlier than 24 hours post-fertilization,” Bertozzi says. “Because many important developmental events including cell migration, tissue morphogenesis, and cell differentiation occur in the first 24 hours of zebrafish embryogenesis, and glycan biosynthesis is also known to occur within that 24 hour period, we sought to develop methods that would enable us to image the glycans early during embryogenesis.”
For this study, Bertozzi and her co-authors microinjected embryos with azido sugars at the one-cell stage, allowed the zebrafish to develop, and then detected the metabolically labeled glycans with the copper-free click chemistry technique. This enabled them to image certain types of glycans as early as seven hours post-fertilization. In addition, they used a complementary, non-metabolic labeling technique to target a class of glycans that carry sialic acid, giving them simultaneous but independent ways to image two distinct classes of glycans.
Time-lapse and multicolor imaging experiments highlighted differences between the O-glycans and sialylated glycans in the cells during the gastrulation and segmentation periods of embryogenesis. The results revealed a dramatic re-organization of cell-surface glycans during mitosis, highlighted by their rapid migration to the cleavage furrow of mitotic cells. A cleavage furrow is the indentation that forms in a mother cell, marking the position where it will divide into two daughter cells.
“As the saying goes, a picture is worth a thousand words,” says Jeremy Baskin, the lead author on this new PNAS paper who is now doing post-doctoral research at Yale University.“In our case, the glycan imaging technology led directly to the observation that membrane-associated glycans traffic laterally within the plasma membrane to the cleavage furrow during cell division. Down the road, we hope to learn the precise molecular structures of the glycans involved in this process and what roles they play.”
Baskin says the ability of this click chemistry technique to provide visualization of specific molecules in cells is an important first step toward an eventual understanding of the function of these molecules.
“By perturbing the system using genetic or pharmacological means and then observing any changes in glycan localization and behavior through imaging,” he says, “we may be able to understand the many functions of glycans in embryogenesis and many other physiological processes, including diseases.”
Bertozzi says she and her colleagues are following up their glycan imaging studies of zebrafish embryos with mechanistic studies that will help determine what role the labeled glycans might play in the process of cell division.
“There are other applications of glycan imaging in mice that we also are aggressively pursuing,” she says, “such as tumor imaging, in addition to studies of how glycans change during embryogenesis.”
This research was funded in part by grants from the National Institutes of Health.
Berkeley Lab is a U.S. Department of Energy (DOE) national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California for the DOE Office of Science. Visit our Website at http://www.lbl.gov.
Additional Information
For more about the research of Carolyn Bertozzi, visit her Website at www.cchem.berkeley.edu/crbgrp/
For more about the research of Sharon Amacher, visit her Website at http://mcb.berkeley.edu/labs/amacher/
