To learn how something works in biology, it pays to start really small. Take this research for example: A team that includes Berkeley Lab scientists has identified and mapped the locations of many of the proteins that compose a hair bundle, which is an organelle that sprouts from hair cells in the inner ear.
Their work brings scientists one step closer to learning how hearing and balance function at the molecular scale. In addition, about 100 of the proteins inventoried by the scientists are encoded by genes implicated in deafness. Insights into the roles of these proteins could lead to a more complete understanding of the causes of hearing loss, and how to fight them.
Hair bundles sway to and fro as the eardrum absorbs sound waves. When this happens, hair cells release neurotransmitters that reach the central nervous system. In this way, the faintest mechanical stimulation—such as sound waves from a pin dropping—is translated into something we hear.
As recently reported, scientists from Oregon Health & Science University used quantitative mass spectrometry to identify more than 1100 proteins in hair bundles obtained from embryonic chicks. Some of the proteins were present in very small numbers, while other proteins such as actin were present by the hundreds of thousands. In all, 336 proteins were present in hair bundles in significant numbers.
But where are these proteins located and what is their role in hearing? Fortunately, answering this question is made easier because the hair bundle is a well-defined organelle with a very specialized function. This means that scientists can get a pretty good idea of a protein’s function by determining its precise location.
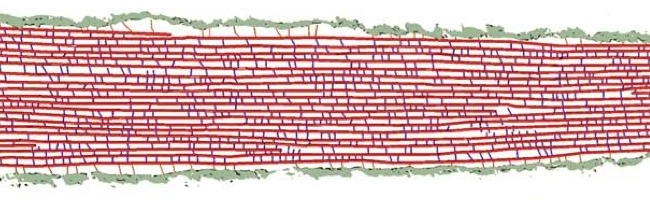
This model, based on 3-D electron tomography images, reveals the 3-D architecture of the actin bundle (red) that compose a stereocilium, the actin-actin cross-linkers (blue), and the linkers (orange) that connect the actin bundle to the membranes (green). Images such as this will help scientists understand the physiology of hearing and balance at a molecular level. For scale, the black bar is 100 nanometers.
With this in mind, Berkeley Lab scientists led by Manfred Auer of the Life Sciences Division used three-dimensional electron tomography to image stereocilia, which are hair-like protrusions that make up hair bundles. Stereocilia are themselves composed of actin filaments that are linked to each other and to the surrounding membrane. The images allowed the scientists to tally the number of actin filaments, cross-linkers, and actin-membrane linkers. It also allowed the scientists to map their positions.
“The two approaches—quantitative proteomics and structural 3-D imaging—independently yielded protein abundance estimates that were remarkably similar,” says Auer. “This demonstrates how imaging can be combined with proteomics to yield mechanistic insights into how biological systems work.”
The scientists next want to map the locations of the 100 or so proteins encoded by genes that are associated with deafness. By combining electron tomography with fluorescent labeling, they can determine the exact positions of these proteins and thus determine their likely function. Knock-out studies can also reveal changes in their architectural organization. This will enable scientists to study the pathogenesis of hearing loss and deafness at a molecular level, which could guide the development of effective therapies.