
Biocrust amongst one of its many natural habitats, taken about 20 miles from the sampling site (near the Corona Arch, Moab, UT). Credit: Tami Swenson
Arid lands, which cover some 40 percent of the Earth’s terrestrial surface, are too dry to sustain much in the way of vegetation. But far from being barren, they are home to diverse communities of microorganisms—including fungi, bacteria, and archaea—that dwell together within the uppermost millimeters of soil. These biological soil crusts, or biocrusts, can exist for extended periods in a desiccated, dormant state. When it does rain, the microbes become metabolically active, setting in motion a cascade of activity that dramatically alters both the community structure and the soil chemistry.
“These biocrusts and other soil microbiomes contain a tremendous diversity of both microbes and small molecules (‘metabolites’). However, the connection between the chemical diversity of soil and microbial diversity is poorly understood,” said Trent Northen, a senior scientist at Lawrence Berkeley National Laboratory (Berkeley Lab).
In a paper published January 2, 2018, in Nature Communications, Berkeley Lab researchers led by the Northen lab report that specific compounds are transformed by and strongly associated with specific bacteria in native biological soil crust (biocrust) using a suite of tools Northen calls “exometabolomics.” Understanding how microbial communities in the biocrusts adapt to their harsh environments could provide important clues to help shed light on the roles of soil microbes in the global carbon cycle.
The work follows a 2015 study that examined how specific small molecule compounds called “metabolites” were transformed in a mixture of bacterial isolates from biocrust samples cultured in a milieu of metabolites from the same soil. “We found that the microbes we investigated were ‘picky’ eaters,” Northen said. “We thought we could use this information to link what’s being consumed to the abundance of the microbes in the intact community, thereby linking the biology to the chemistry.”
In the new study, the investigators set out to determine whether the microbe-metabolite relationships observed in the simplified test-tube system could be reproduced in a more complex soil environment.
Biocrusts from the same source – representing four successive stages of maturation – were wet, and the soil water was sampled at five time points. The samples were analyzed by liquid chromatography-mass spectrometry (LC-MS) to characterize the metabolite composition (“metabolomics”), and biocrust DNA was extracted for shotgun sequencing to measure single copy gene markers for the dominant microbe species (“metagenomics”).

Biocrust is held together primarily by exopolysaccharides produced by the filamentous Cyanobacterium, M. vaginatus. Samples from the field were collected in petri dishes. In the lab, they were removed from the dishes, cut and placed into multi-well plates before adding water. Credit: Tami Swenson
“When we compare the patterns of metabolite uptake and production for isolated bacteria that are related to the most abundant microbes found in the biocrusts, we find that, excitingly, these patterns are maintained,” said Northen. That is, increased abundance of a given microbe is negatively correlated with the metabolites that they consume and positively correlated with metabolites that they release.
When active, biocrusts take up atmospheric carbon dioxide and fix nitrogen, contributing to the ecosystem’s primary productivity. They also process organic matter in soil, modifying key properties related to soil fertility and water availability.
“This study suggests that laboratory studies of microbial metabolite processing can help understand the role of these microbes in carbon cycling in the environment. This study gets us closer to understanding the complex food webs that are vital in nutrient dynamics and overall soil fertility,” said study first author Tami Swenson, a scientific engineering associate in Northen’s group within the Berkeley Lab Biosciences Area’s Environmental Genomics and Systems Biology (EGSB) Division.
Northen’s group is currently working on expanding these studies to capture a greater fraction of microbial diversity. Ultimately, this may enable the prediction of nutrient cycling in terrestrial microbial ecosystems, and perhaps even manipulation by adding specific metabolites.
The following Berkeley Lab researchers also contributed to the study: Benjamin Bowen, a member of Northen’s lab in EGSB and at the Joint Genome Institute, a DOE Office of Science User Facility, helped analyze metabolomics data; Ulas Karaoz in the Earth and Environmental Sciences Area (EESA) analyzed metagenomics data; and Joel Swenson, a former postdoctoral researcher in Biosciences’ Biological Systems and Engineering Division, helped conduct correlation and statistical analyses.
This work was supported under a DOE Office of Science Early Career Research Program award. DNA was sequenced using the Vincent J. Coates Genomics Sequencing Laboratory at UC Berkeley, supported by a National Institutes of Health Instrumentation Grant.
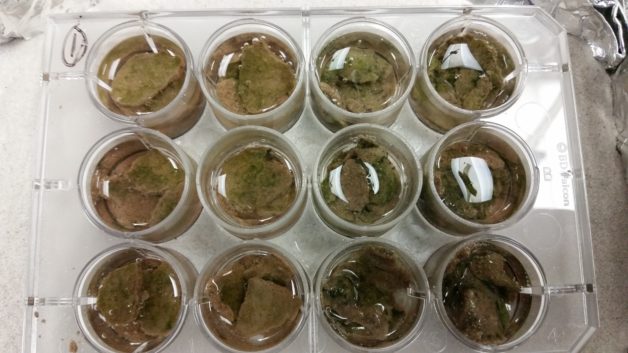
Regular water was added to mimic a rainfall event. The microbes in biocrust become metabolically active immediately upon wetting. As seen here, M. vaginatus turns green and releases oxygen. Credit: Tami Swenson
# # #
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel Prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit the Office of Science website at science.energy.gov/.
