
In just 10 years, scientists at Berkeley Lab and the Joint Center for Artificial Photosynthesis have pioneered major advances in solar fuels technologies. (Credit: Gencho Petkov/Shutterstock)
Since its founding in 2010, the Joint Center for Artificial Photosynthesis (JCAP) has made significant progress in the pursuit of a sustainable energy industry that converts sunlight, water, and carbon dioxide into renewable transportation fuels. JCAP is a U.S. Department of Energy (DOE) Innovation Hub led by the California Institute of Technology (Caltech) and based partly at DOE’s Lawrence Berkeley National Laboratory (Berkeley Lab).
As we look back at a decade of discovery, we highlight 10 scientific breakthroughs by researchers at Berkeley Lab, Caltech’s lead partner. All of these advances – from designing new materials that convert carbon dioxide into sustainable chemicals and fuels without wasteful byproducts, to sophisticated devices that transform sunlight and water into electricity and fuels that could replace coal, oil, and other fossil fuels – point to Berkeley Lab’s progress toward a solar fuels future.
1. A Solar Cell That Does Double Duty for Renewable Energy
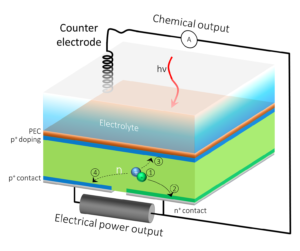
Schematic of a hybrid photoelectrochemical and voltaic cell that converts sunlight and water into hydrogen fuel and electricity. (Credit: Berkeley Lab)
Researchers at Berkeley Lab and JCAP developed an artificial photosynthesis device that turns sunlight and water into two types of energy: hydrogen fuel and electricity. Full story here.
2. Greener Days Ahead for Carbon Fuels

At JCAP, Yanwei Lum (left) and Joel Ager discovered copper’s potential as a clean catalyst for turning CO2 into sustainable chemicals and fuels. (Credit: Marilyn Chung/Berkeley Lab)
Researchers at Berkeley Lab and JCAP demonstrated copper’s potential as a catalyst for turning carbon dioxide into sustainable chemicals and fuels without any wasteful byproducts, creating a green alternative to present-day chemical manufacturing. Full story here.
3. A Chemical Reaction Close-Up: New Technology Gives a Glimpse of Solar Fuel Generation in Action
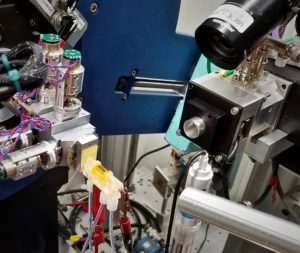
The team’s electrochemical cell for observing solar fuel-generating catalysts (yellow device), set up at an x-ray beamline at the Stanford Synchrotron Radiation Lightsource. (Credit: Walter Drisdell/Berkeley Lab)
JCAP scientists invented a cell that is expected to help scientists engineer and test new catalyst materials, which can be used in next-generation solar fuel devices that split water to produce hydrogen gas and convert carbon dioxide emissions into fuels like ethanol. Full story here.
4. Here Comes the Sun: A New Framework for Artificial Photosynthesis
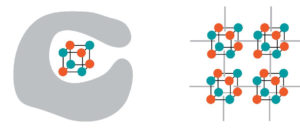
To hold the catalysts in place, the researchers used a metal-organic framework as a scaffold. (Credit: Andy Nguyen et al./Berkeley Lab)
Scientists have long sought to mimic the process by which plants make their own fuel using sunlight, carbon dioxide, and water through artificial photosynthesis devices, but how exactly substances called catalysts work to generate renewable fuel remains a mystery. In work led by Berkeley Lab and JCAP, scientists gained new insight into how to better control cobalt oxide, one of the most promising catalysts for artificial photosynthesis. Full story here.
5. Solar-to-Fuel System Recycles CO2 to Make Ethanol and Ethylene
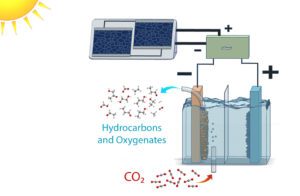
Schematic of a solar-powered electrolysis cell which converts carbon dioxide into hydrocarbon and oxygenate products. (Credit: Clarissa Towle/Berkeley Lab)
Scientists at Berkeley Lab and JCAP successfully demonstrated the approach of going from carbon dioxide directly to target products, namely ethanol and ethylene, at energy conversion efficiencies rivaling natural counterparts. They did this by optimizing each component of a photovoltaic-electrochemical system to reduce voltage loss, and creating new materials when existing ones did not suffice. Full story here.
6. Splitting Water: Nanoscale Imaging Yields Key Insights

Berkeley Lab researchers Francesca Toma (left) and Johanna Eichhorn used a photoconductive atomic force microscope to better understand materials for artificial photosynthesis. (Credit: Marilyn Chung/Berkeley Lab)
Researchers at Berkeley Lab and JCAP pioneered a technique that uses nanoscale imaging to understand how local, nanoscale properties in a material can affect its ability to efficiently perform photoelectrochemical water splitting, and also to understand why a certain material may or may not work in an artificial photosynthesis system. Full story here.
7. New Materials Could Turn Water into the Fuel of the Future

Researchers are using a new high-throughput method of identifying new materials. (Photo Credit: Caltech)
Scientists at Berkeley Lab and Caltech nearly doubled the number of materials known to have potential for use in solar fuels. They did so by developing a process that promises to speed the discovery of commercially viable solar fuels that could replace coal, oil, and other fossil fuels. Full story here.
8. Solar Cells Get Boost with Integration of Water-Splitting Catalyst onto Semiconductor

Schematic of the multi-functional water splitting catalyst layer engineered using atomic layer deposition. (Credit: Ian Sharp/Berkeley Lab)
JCAP scientists devised a method for engineering a composite film that supports chemical reactions without damaging sensitive semiconductors in an artificial photosynthesis system designed to generate clean fuel. Full story here.
9. New Discovery Could Better Predict How Semiconductors Weather Abuse

Berkeley Lab scientists at JCAP are working to improve systems that efficiently convert sunlight, water and carbon dioxide into fuel. Shown (left to right) are David Larson, Kristin Persson, Jeff Beeman, Ian Sharp, and Francesca Toma. (Credit: Paul Mueller/Berkeley Lab)
One of the major challenges for scientists working to create systems that efficiently convert sunlight, water, and carbon dioxide into fuel is finding materials that can do the work while also surviving the corrosive conditions that are part of the process. Existing methods to determine material stability have been hit and miss, but a Berkeley Lab-led team of researchers at JCAP applied a combination of experimental and theoretical tools to rigorously determine how well a material will weather the harsh environments in these systems. Full story here.
10. Some Like it Hot: Simulating Single Particle Excitations
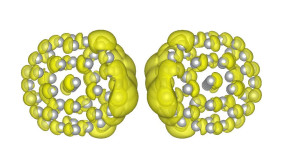
Changes in charge density ‘slosh‘ from one side to the other within the nanoparticle. (Credit: Berkeley Lab)
Plasmons – clouds of electrons that oscillate within a cluster of metal nanoparticles – could serve as antennae in a photovoltaic device to absorb sunlight more efficiently than semiconductors. To leverage plasmons for a solar-cell application, scientists at Berkeley Lab used a real-time algorithm to simulate how electrons move within each nanoparticle when a plasmon is excited by light. The JCAP-supported study provided a new understanding of how long a particle is excited by light in a solar fuels device. Full story here.
###
Founded in 1931 on the belief that the biggest scientific challenges are best addressed by teams, Lawrence Berkeley National Laboratory and its scientists have been recognized with 13 Nobel Prizes. Today, Berkeley Lab researchers develop sustainable energy and environmental solutions, create useful new materials, advance the frontiers of computing, and probe the mysteries of life, matter, and the universe. Scientists from around the world rely on the Lab’s facilities for their own discovery science. Berkeley Lab is a multiprogram national laboratory, managed by the University of California for the U.S. Department of Energy’s Office of Science.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit energy.gov/science.
