A team from Lawrence Berkeley National Laboratory (Berkeley Lab) and Florida State University has designed a new blueprint for solid-state batteries that are less dependent on specific chemical elements, particularly critical metals that are challenging to source due to supply chain issues. Their work, reported recently in the journal Science, could advance solid-state batteries that are efficient and affordable.
Touted for their high energy density and superior safety, solid-state batteries could be a game-changer for the electric car industry. But developing one that is affordable and also conductive enough to power a car for hundreds of miles on a single charge has long been a challenging hurdle to overcome.
“With our new approach to solid-state batteries, you don’t have to give up affordability for performance.” – Yan Zeng, Berkeley Lab staff scientist, Materials Sciences Division

Yan Zeng, Berkeley Lab staff scientist (Credit: courtesy of Yan Zeng)
“Our work is the first to solve this problem by designing a solid electrolyte with not just one metal but with a team of affordable metals,” said co-first author Yan Zeng, a staff scientist in Berkeley Lab’s Materials Sciences Division.
In a lithium-ion battery, the electrolyte works like a transfer hub where lithium ions move with electric charge to either power a device or recharge the battery.
Like other batteries, solid-state batteries store energy and then release it to power devices. But rather than liquid or polymer gel electrolytes found in lithium-ion batteries, they use a solid electrolyte.
Government, research, and academia have heavily invested in the research and development of solid-state batteries because the liquid electrolytes designed for many commercial batteries are more prone to overheating, fire, and loss of charge.
However, many of the solid-state batteries constructed thus far are based on specific types of metals that are expensive and not available in large quantities. Some aren’t found at all in the United States.
For the current study, Zeng – along with Bin Ouyang, an assistant professor in chemistry and biochemistry at Florida State University – and senior author Gerbrand Ceder, a Berkeley Lab faculty senior scientist and UC Berkeley professor of materials science and engineering, demonstrated a new type of solid electrolyte consisting of a mix of various metal elements. Zeng and Ouyang first developed the idea for this work while finishing their postdoctoral research at Berkeley Lab and UC Berkeley under the supervision of Ceder.
The new materials could result in a more conductive solid electrolyte that is less dependent on a large quantity of an individual element.
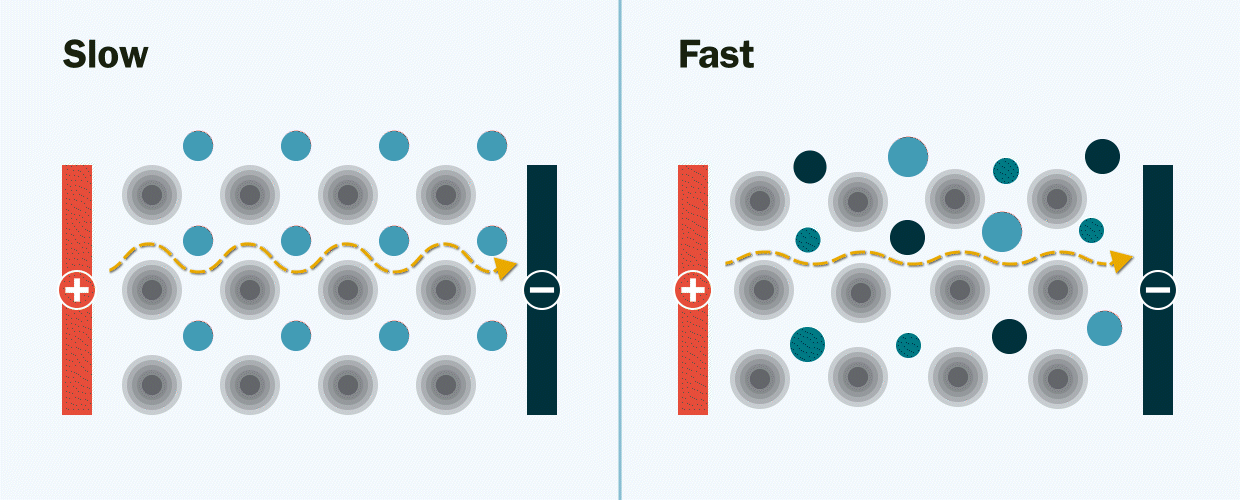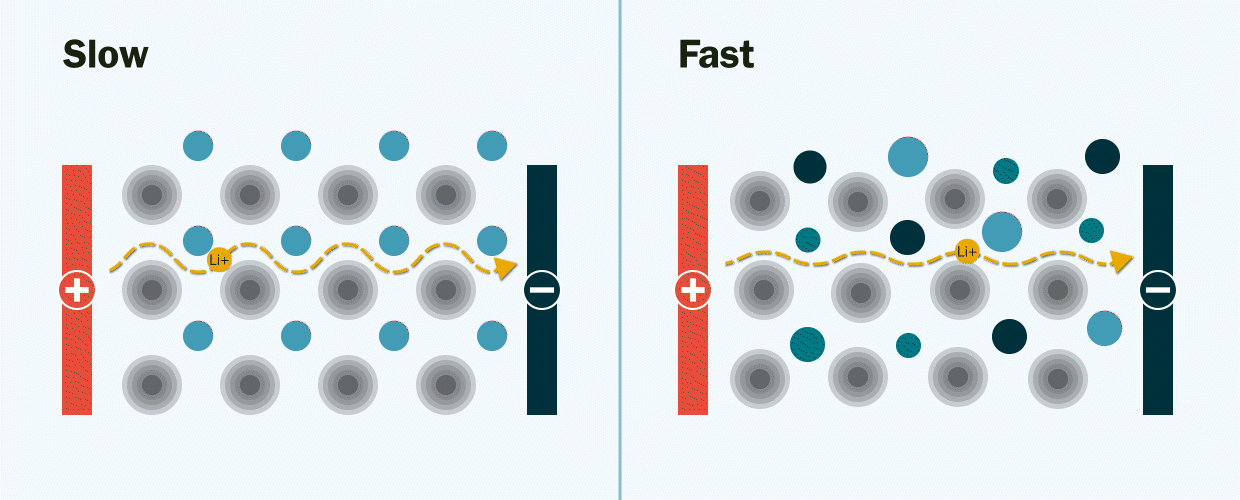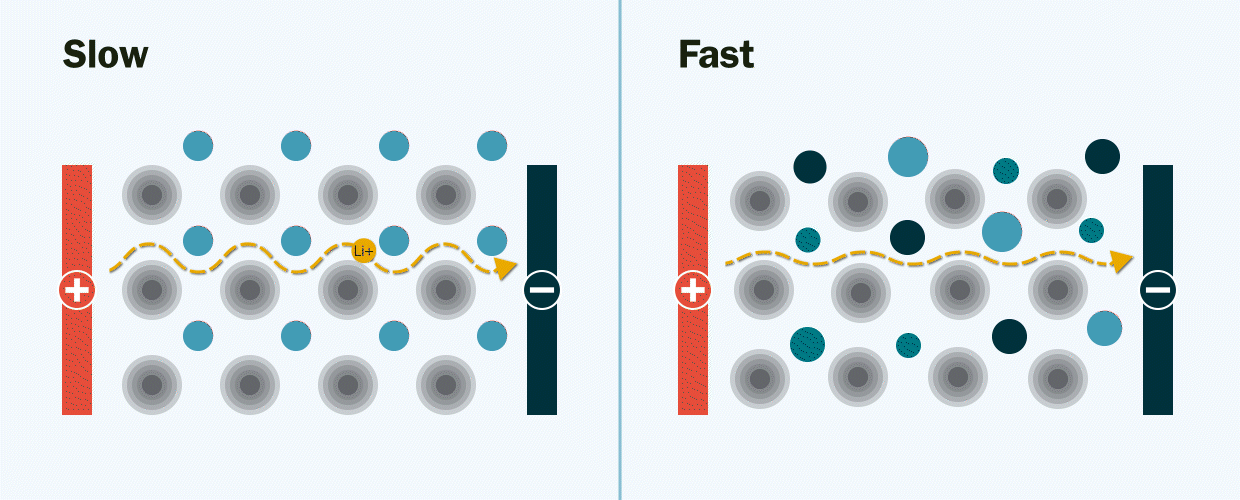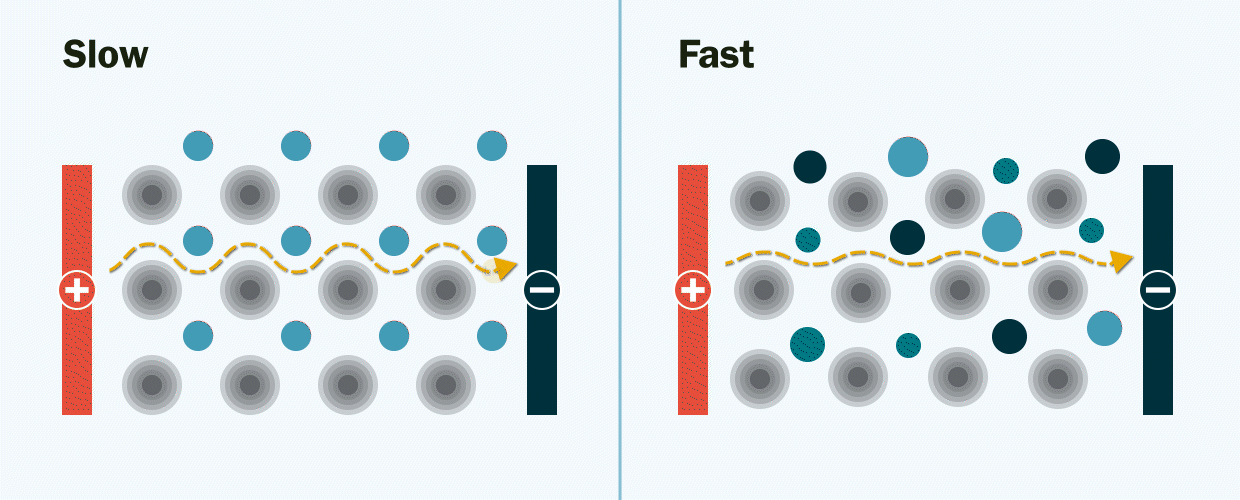
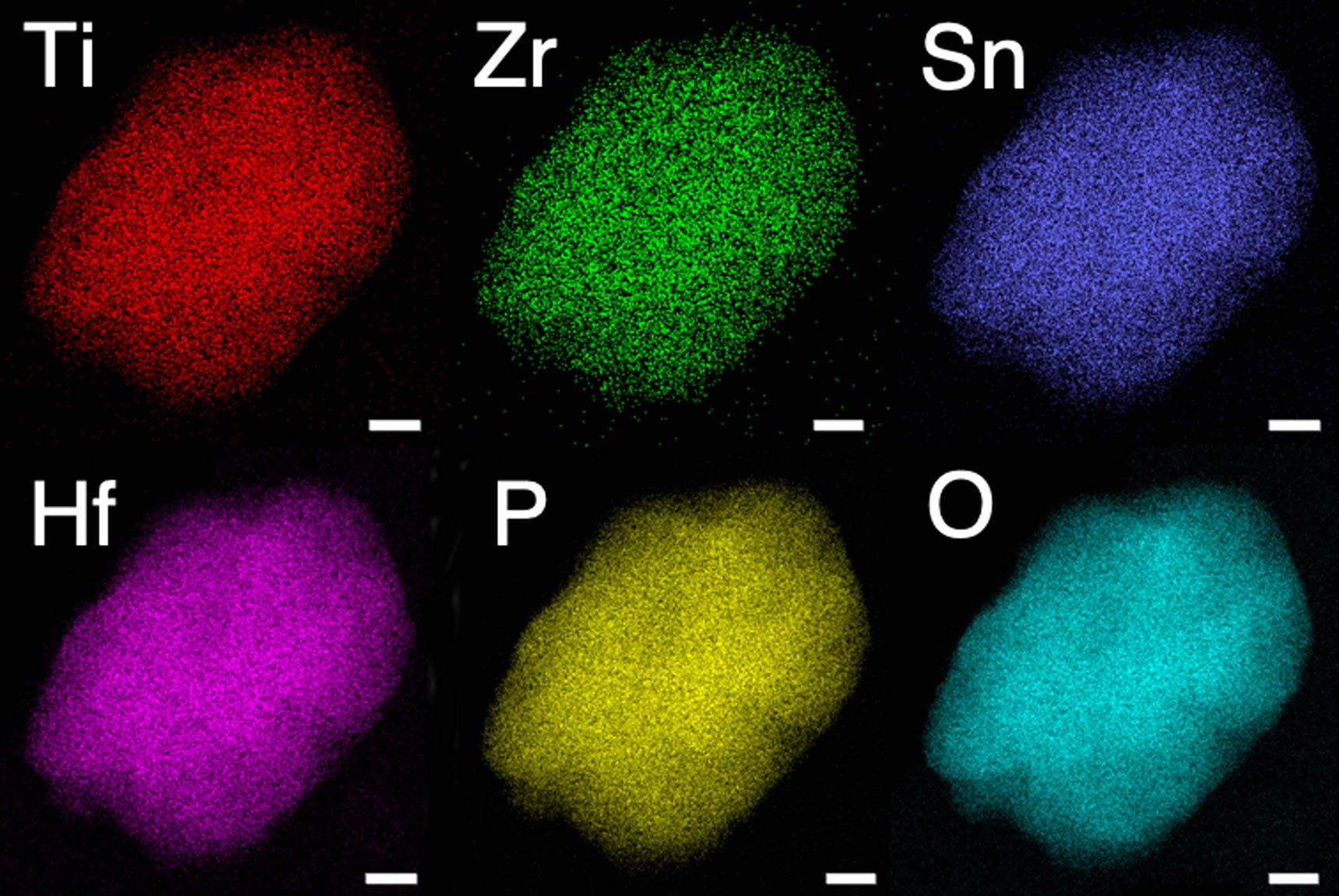
In experiments at Berkeley Lab and UC Berkeley, the researchers demonstrated the new solid electrolyte by synthesizing and testing several lithium-ion and sodium-ion materials with multiple mixed-metals.
They observed that the new multi-metal materials performed better than expected, displaying an ionic conductivity several orders of magnitude faster than the single-metal materials. Ionic conductivity is a measurement of how quickly lithium ions move to conduct electric charge.
The researchers theorize that mixing many different types of metals together creates new pathways – much like the addition of expressways on a congested highway – through which lithium ions can move quickly through the electrolyte. Without these pathways, the movement of lithium ions would be slow and limited when they travel through the electrolyte from one end of the battery to the other, Zeng explained.
To validate candidates for the multi-metal design, the researchers performed advanced theoretical calculations based on a method called density-functional theory on supercomputers at the National Energy Research Scientific Computing Center (NERSC). Using scanning transmission electron microscopes (STEM) at the Molecular Foundry, the researchers confirmed that each electrolyte is made of only one type of material – what scientists call a “single phase” – with unusual distortions giving rise to the new ion transport pathways in its crystal structure.
The discovery enables new opportunities to design next-generation ionic conductors. The next step in this research is to apply the new approach that Zeng has developed with Ceder at Berkeley Lab to further explore and discover novel solid electrolyte materials that can improve battery performance even further.
This work represents one of the many ways in which experts at the Berkeley Lab Energy Storage Center are working to enable the nation’s transition to a clean, affordable, and resilient energy future.
Last year, Ouyang won a NERSC High Performance Computing Achievement Award for “advancing the understanding of chemical short-range order for designing a new generation of commercialized cathode materials.” The award recognizes early-career scientists who have made significant contributions to scientific computation using NERSC resources.
Other scientists contributing to this work are Young-Woon Byeon and Zijian Cai from Berkeley Lab, Jue Liu from Oak Ridge National Laboratory, and Lincoln Miara and Yan Wang from the Samsung Advanced Institute of Technology.
The Molecular Foundry and NERSC are DOE Office of Science user facilities at Berkeley Lab.
This research was supported by the DOE Vehicle Technologies Office.
# # #
Founded in 1931 on the belief that the biggest scientific challenges are best addressed by teams, Lawrence Berkeley National Laboratory and its scientists have been recognized with 16 Nobel Prizes. Today, Berkeley Lab researchers develop sustainable energy and environmental solutions, create useful new materials, advance the frontiers of computing, and probe the mysteries of life, matter, and the universe. Scientists from around the world rely on the Lab’s facilities for their own discovery science. Berkeley Lab is a multiprogram national laboratory, managed by the University of California for the U.S. Department of Energy’s Office of Science.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit energy.gov/science.

On the Road to Better Solid-State Batteries

On the Road to Better Solid-State Batteries