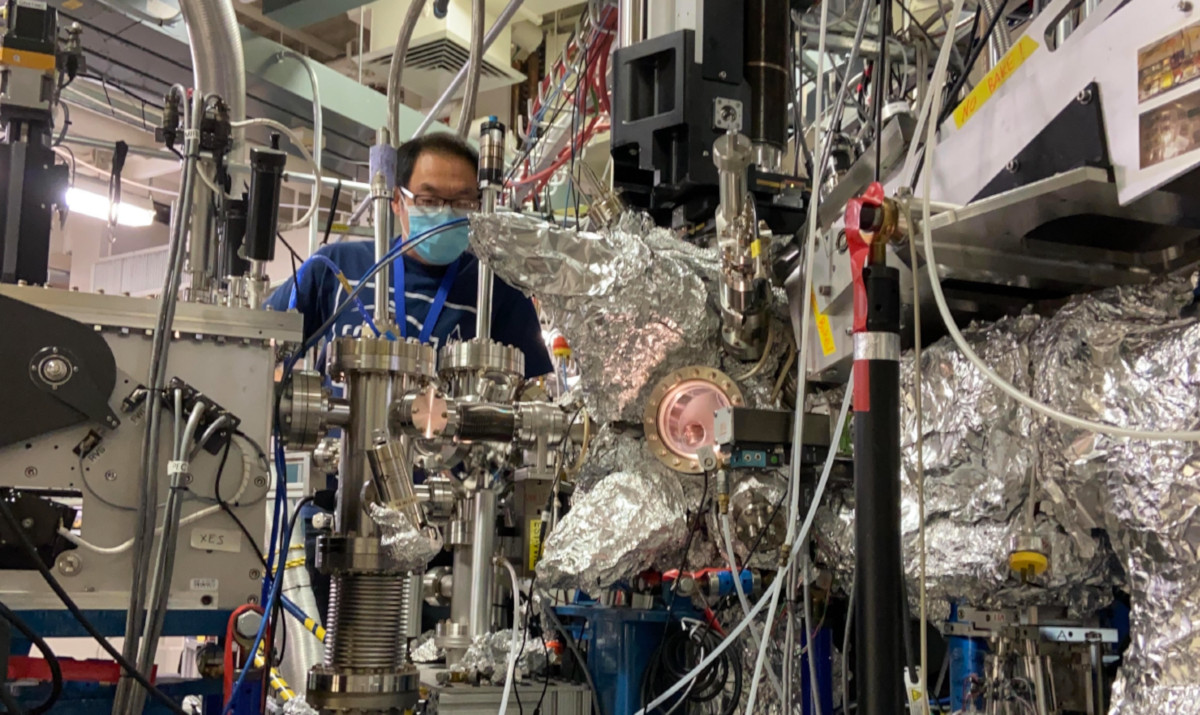
Scientists at Lawrence Berkeley National Laboratory (Berkeley Lab) have developed a conductive polymer coating – called HOS-PFM – that could enable longer lasting, more powerful lithium-ion batteries for electric vehicles.
“The advance opens up a new approach to developing EV batteries that are more affordable and easy to manufacture,” said Gao Liu, a senior scientist in Berkeley Lab’s Energy Technologies Area who led the development of the material.
The HOS-PFM coating conducts both electrons and ions at the same time. This ensures battery stability and high charge/discharge rates while enhancing battery life. The coating also shows promise as a battery adhesive that could extend the lifetime of a lithium-ion battery from an average of 10 years to about 15 years, Liu added.
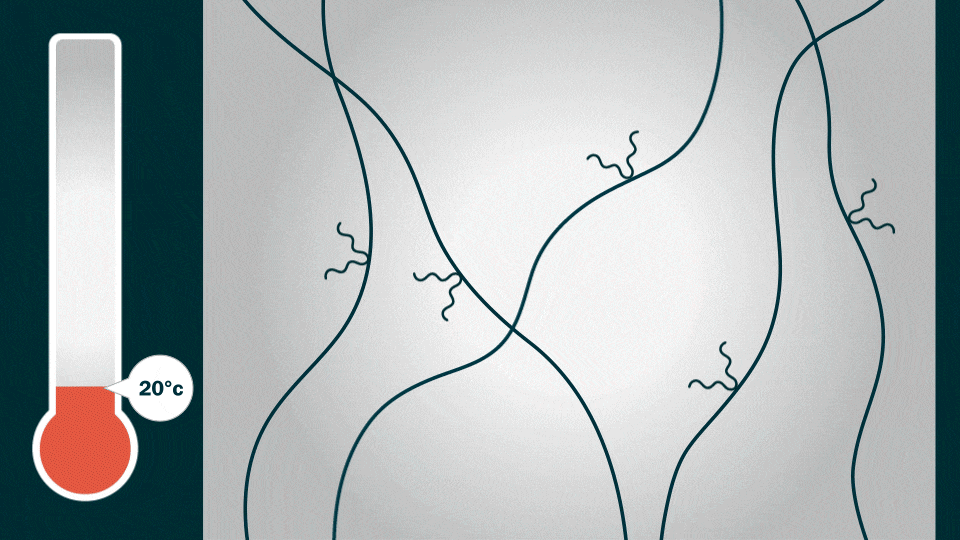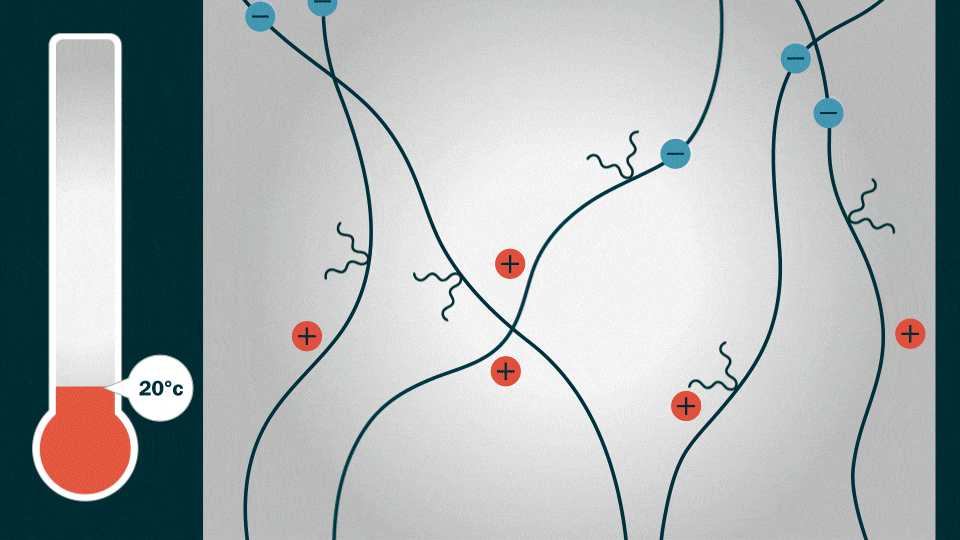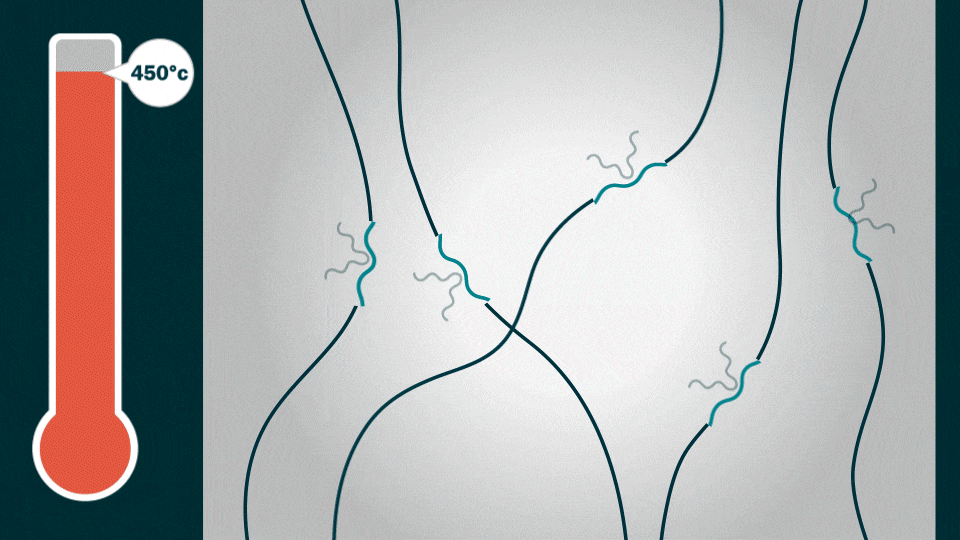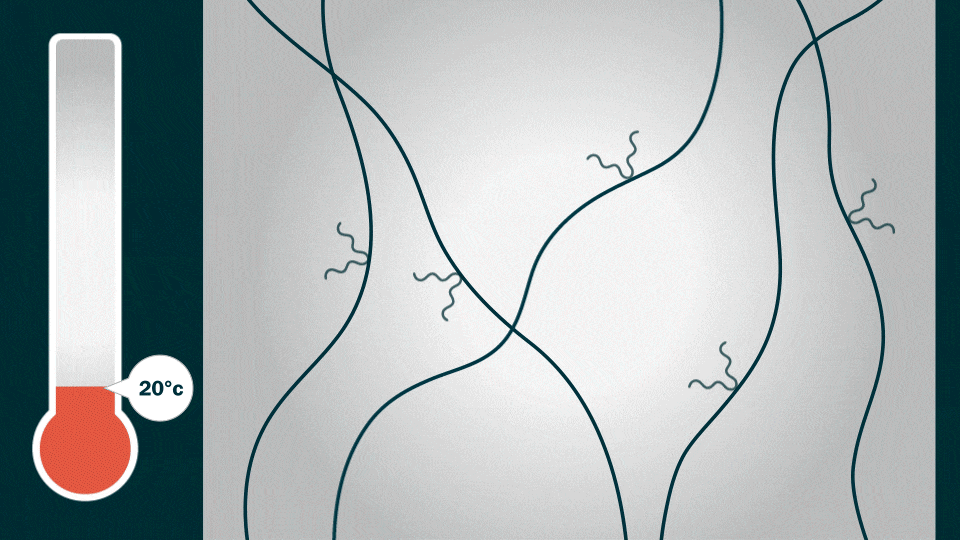
To demonstrate HOS-PFM’s superior conductive and adhesive properties, Liu and his team coated aluminum and silicon electrodes with HOS-PFM, and tested their performance in a lithium-ion battery setup.
Silicon and aluminum are promising electrode materials for lithium-ion batteries because of their potentially high energy storage capacity and lightweight profiles. But these cheap and abundant materials quickly wear down after multiple charge/discharge cycles.
During experiments at the Advanced Light Source and the Molecular Foundry, the researchers demonstrated that the HOS-PFM coating significantly prevents silicon- and aluminum-based electrodes from degrading during battery cycling while delivering high battery capacity over 300 cycles, a performance rate that’s on par with today’s state-of-the-art electrodes.
The results are impressive, Liu said, because silicon-based lithium-ion cells typically last for a limited number of charge/discharge cycles and calendar life. The researchers recently described these findings in the journal Nature Energy.
“The advance opens up a new approach to developing EV batteries that are more affordable and easy to manufacture.”– Gao Liu, Berkeley Lab senior scientist, Energy Technologies Area
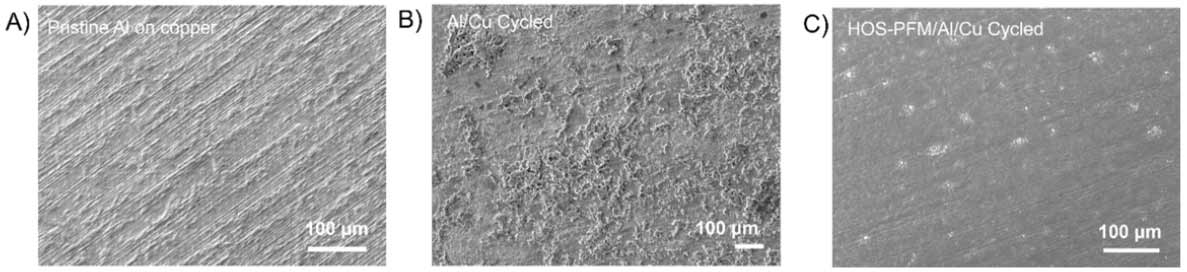
The HOS-PFM coating could allow the use of electrodes containing as much as 80% silicon. Such high silicon content could increase the energy density of lithium-ion batteries by at least 30%, Liu said. And because silicon is cheaper than graphite, the standard material for electrodes today, cheaper batteries could significantly increase the availability of entry-level electric vehicles, he added.
The team next plans to work with companies to scale up HOS-PFM for mass manufacturing.
The Advanced Light Source and Molecular Foundry are DOE Office of Science user facilities at Berkeley Lab.
The research was supported by DOE Vehicle Technologies Office. Additional funding was provided by the Toyota Research Institute. The technology is available for licensing by contacting [email protected].
# # #
Founded in 1931 on the belief that the biggest scientific challenges are best addressed by teams, Lawrence Berkeley National Laboratory and its scientists have been recognized with 16 Nobel Prizes. Today, Berkeley Lab researchers develop sustainable energy and environmental solutions, create useful new materials, advance the frontiers of computing, and probe the mysteries of life, matter, and the universe. Scientists from around the world rely on the Lab’s facilities for their own discovery science. Berkeley Lab is a multiprogram national laboratory, managed by the University of California for the U.S. Department of Energy’s Office of Science.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit energy.gov/science.

Electric Vehicle Batteries Could Get Big Boost With New Polymer Coating
Electric Vehicle Batteries Could Get Big Boost With New Polymer Coating