Paul Preuss, (510) 486-6249, [email protected]
Oxygen is the most abundant element in Earth’s crust and accounts for almost a third the planet’s mass. Of its three stable isotopes, oxygen 16 (whose nucleus contains eight neutrons) makes up 99.762 percent of oxygen on Earth, while heavier oxygen 17 (with nine neutrons) accounts for just 0.038 percent, and the heaviest isotope, oxygen 18 (with 10 neutrons), makes up 0.2 percent.

After breaking into thousands of fragments in the atmosphere, the Allende meteorite scattered widely across Chihuahua, Mexico. It contains some of the oldest minerals formed in the solar system. (Inset photo, “a puzzled Earthman,” courtesy Brian Mason, Smithsonian Institution)
Yet minerals in some of the most primitive objects in the solar system, including the meteorites called carbonaceous chondrites, have quite different ratios of oxygen isotopes than on Earth; presumably the rare heavy isotopes occurred in much greater abundances in the early solar system.
“For a chemist, the question of oxygen-isotope ratios is one that could help us understand the origins of the solar system,” says Musahid (Musa) Ahmed of Berkeley Lab’s Chemical Sciences Division, a beamline scientist at the Chemical Dynamics beamline, 9.0.2, at the Advanced Light Source (ALS). “Why meteoritic oxygen isotope ratios are significantly different from those on Earth has mystified scientists for years.”
Various models have been proposed to explain these differences, including the notion that isotope ratios in our solar system resulted from their creation in an exotic star, or in several different stars, through nuclear processes – models that Ahmed says “don’t work” – or, more persuasively, that chemical processes within the solar nebula itself gave rise to the oxygen ratios.
One such process goes by the name of “isotope self-shielding.” The most abundant oxygen-bearing molecule in the solar nebula was carbon monoxide, CO, and self-shielding has been considered a key to the relative amounts of oxygen that result when CO is dissociated by vacuum ultraviolet light, or VUV. (VUV refers to wavelengths from 200 to 10 nanometers.)
Self-shielding has been observed in molecular clouds of dust and gas in outer space. When energetic VUV light from a nearby star penetrates a molecular cloud, it breaks CO molecules into atoms of carbon and oxygen. Different isotopes absorb VUV photons with slightly different energies, however; near the edge of the cloud, the CO with the most abundant isotope, 16O, soaks up many of the photons that can be absorbed by 16O, thus shielding 16O deeper in the cloud. But 17O and 18O, which absorb different energies, are not shielded. Inside the cloud, then, relatively more CO molecules with the heavier isotopes are dissociated, and heavier oxygen atoms are released.

The sun in ultraviolet light. When the solar system was forming, the protosun was a potent source of vacuum ultraviolet.
It’s reasonable to expect that a similar process may have been at work in the early solar system, with the young sun radiating VUV that acted on CO in a hot region near the protosun, or perhaps in colder regions farther away. Does VUV self-shielding really work under these conditions? And if so, what effect does it have on the resulting ratio of oxygen isotopes? Until now there were no answers; the proposal had never been experimentally tested.
“Mark Thiemens of the University of California at San Diego contacted us to use beamline 9.0.2 to do a direct test,” says Ahmed. “The ALS provides VUV photons that can be tuned precisely to various energies that dissociate CO.”
Cosmochemist Thiemens has investigated the question of oxygen ratios in the solar system for over thirty years; he is a member of the science team for the Genesis spacecraft that returned samples from the solar wind. He believes that how the solar system formed and evolved cannot be understood without understanding the cosmochemistry of oxygen.
Along with CO photodissociation in the early solar system, water is also a key player in the process. Together they make for some intricate chemistry, which locks the heavier isotopes of oxygen into minerals that make up the oldest meteorites and subsequently formed all the other bodies of the solar system.
“The first step is the photodissociation of CO into carbon and oxygen atoms,” Ahmed explains. “The oxygen combines with a hydrogen atom to form hydroxyl, OH, a radical which quickly picks up another hydrogen atom to form water, H2O. All of this is presumably mediated on a dust particle. Thus the oxygen in the water, some models suggest, transfers its isotopic signature to silicates. The different oxygen isotopes persist through these steps; our experiment looked at what happens in the first step.”
The experimenters sent ultrahigh-purity carbon monoxide through a test chamber and exposed each run to a beam of VUV photons generated in a synchrotron at four different wavelengths that were important for the self-shielding hypothesis. Exposure time at each wavelength was long, from a little over three hours to almost 16 hours.
As the carbon and oxygen atoms dissociated, the oxygen quickly recombined with intact CO to form carbon dioxide, CO2, which was collected in a liquid-nitrogen-cooled vessel. These samples were taken to UC San Diego by team member Subrata Chakraborty, a postdoc in the Thiemens group and lead author of the paper describing the research results. Chakraborty chemically removed O2 from the CO2. He then determined the isotope ratios by mass spectrometry, which separates isotopes according to their mass.
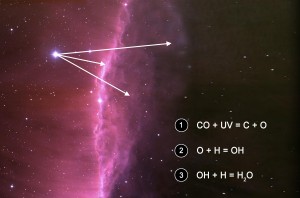
Self-shielding has been observed in molecular clouds but may not account for differing ratios of oxygen isotopes. However they are determined, oxygen isotope ratios are preserved as the oxygen dissociation products of CO combine with hydrogen to form hydroxyl and then water, which later reacts with dust grains to form minerals.
“The results surprised us,” Ahmed said. “We set out to prove that VUV self-shielding is responsible for the ratios of oxygen isotopes characteristic of the oldest objects in the solar system, but it turned out we didn’t need self-shielding.”
While the researchers found differences in the way the CO responded to the different wavelengths used in the experiment, these were not the differences predicted by the self-shielding hypothesis; instead, they were due to electronic properties of the CO molecules that were unrelated to self-shielding.
Basic chemical physics alone was enough to produce the higher proportion of heavier isotopes – and the ratios themselves were still a good match for those found in samples from the early solar system. The authors concluded that cold regions of the solar nebula were indeed a potential site for the generation of oxygen reservoirs with relatively high amounts of the heavier oxygen isotopes, “but not via self-shielding.”
“You can see the ratios of the isotopes brought back by Genesis, but that doesn’t tell you how they came about,” says Ahmed. “The isotope ratios themselves don’t tell you why they were different in the early universe than they are today, so there’s lots more science to do in the laboratory. One of the steps in the chemistry of oxygen that we want to test next is the reaction between oxygen, water and silicates, which produced the solar system’s first rocks. It’s the kind of experiment that beamline 9.0.2 was designed to perform: investigate chemistry in environments like those in interstellar space and in our own earth in combustion engines and the terrestrial atmosphere.”
“Experimental test of self-shielding in vacuum ultraviolet photodissociation of CO,” by Subrata Chakraborty, Musahid Ahmed, Teresa L. Jackson, and Mark H. Thiemens, appears in Science and is available online to subscribers at http://www.sciencemag.org/cgi/content/full/321/5894/1328. The work was supported by NASA and the U.S. Department of Energy.
Additional information
- More about the Genesis mission and the origin of the solar system is at http://genesismission.jpl.nasa.gov/index.html.
- More about varieties of isotopes in the solar system is at http://genesismission.jpl.nasa.gov/gm2/news/features/isotopes.htm
- An interview with Mark Thiemens is at http://genesismission.jpl.nasa.gov/gm2/team/people/thiemans/interview1.htm.
- More about beamline 9.0.2 at the Advanced Light Source, the Chemical Dynamics Beamline, is at http://www.chemicaldynamics.lbl.gov/.