A team of scientists from Lawrence Berkeley National Laboratory (Berkeley Lab) and UC Berkeley have devised a new technique for recycling polyethylene plastic bags and food packaging into propylene or propene gas, a valuable starter material for commercial plastics and commodity chemicals.
Recycling polyethylene plastic into new, recyclable plastics could help reduce carbon emissions linked to plastic pollution and landfill.
“The world produces more than 200 billion pounds of polyethylene,” said scientist John Hartwig, the senior author of the Science study reporting the new technique. “We depend on polyethylenes. They have great benefits for a huge range of applications from pipes and building materials to food preservation. The waste forms of these plastics could be a source of carbon, but these materials are very challenging to recycle. Our work addresses this problem by offering a new strategy for recycling polyethylenes to make chemicals we usually get from fossil sources.”
Hartwig is a senior faculty scientist in the Chemical Sciences Division at Berkeley Lab, and professor of chemistry at UC Berkeley. He is also the Catalysis and Chemical Transformations Program Leader at Berkeley Lab.
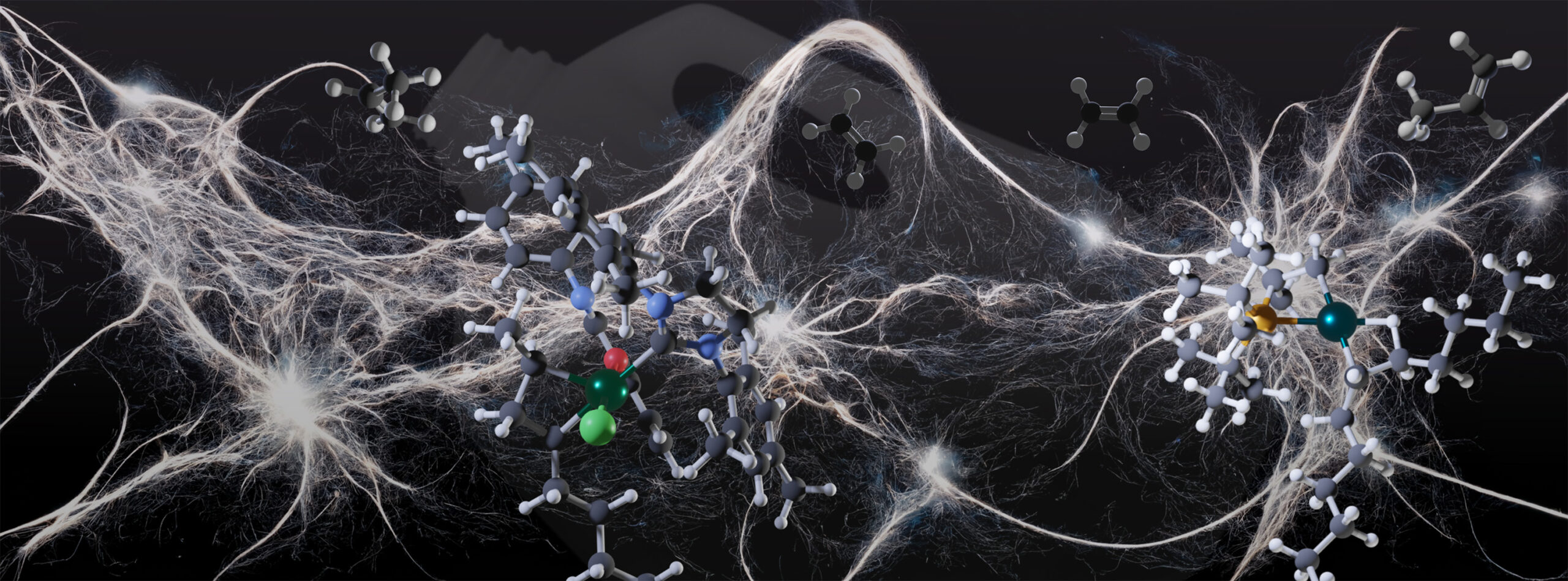
Polyethylene is a polymer chain of molecules or monomers comprising two carbon atoms and four hydrogen atoms: C2H4. For many years, researchers have wanted to find a way to chemically recycle polyethylene by recovering the monomers to make new polyethylene polymers as an alternative to a recycling technique called pyrolysis, which cleaves the chains of polyethylene at very high temperatures to make a mixture of chains. The problem with pyrolysis is that it’s hard to separate the products with a specific chain length you would want for a specific purpose (like for lubricants or as precursors to fuels) from the products that are too short or too long to be used.
So in order to chemically recycle polyethylene, you need to break its chemical bonds. Polyethylene is held together by unusually unreactive carbon-carbon bonds that are very hard to break, but, according to Hartwig, “We have found a way to use a series of catalysts to very selectively cleave those bonds in polyethylene to make propylene, a feedstock chemical.”
In a key experiment, Hartwig and his team dissolved samples of high-density polyethylene (HDPE) (the plastic in container tops, milk jugs, and shampoo bottles) with ethylene gas and a catalyst in a solvent in a pressurized vessel. These conditions, the researchers predicted, would force hydrogen from some of the monomer units – And such hydrogen loss, they reasoned, would in turn enable a series of reactions between the dehydrogenated polymer and ethylene in the presence of additional catalysts to produce propylene.
To the researchers’ surprise, even though hydrogen was removed from just 1.9% of the monomer units, 87% of the carbon atoms in an HDPE polymer chain reacted with ethylene and became propylene in a mere 18 hours. This means that every other monomer in a chain of 1,000 monomer units turned into propylene gas – in other words, “There was no polymer left,” Hartwig said. “I was pretty shocked that it worked so well.”
Experiments at Berkeley Lab’s Molecular Foundry confirmed that changes to the material’s molecular weight during the reactions were occurring as designed.
Hartwig said that although the technique is not yet ready for deployment at an industrial scale, their findings have important implications for recycling polyethylene plastic into carbon feedstocks for new plastics, industrial lubricants, jet fuels, and feedstock chemicals.
In future experiments, he and his team plan to improve the technique’s commercial viability with recyclable catalysts. They would also like to use this work to lay the groundwork for designing new types of chemically recyclable plastic.
This work was supported by the DOE Office of Science.
The Molecular Foundry is a DOE Office of Science nanoscience user facility located at Berkeley Lab.
The technology (UC Case 2022-099) is available for licensing through UC Berkeley’s Office of Technology Licensing.
More:
“Process converts polyethylene bags, plastics to polymer building blocks,” UC Berkeley news release, Sept. 29, 2022
# # #
Founded in 1931 on the belief that the biggest scientific challenges are best addressed by teams, Lawrence Berkeley National Laboratory and its scientists have been recognized with 14 Nobel Prizes. Today, Berkeley Lab researchers develop sustainable energy and environmental solutions, create useful new materials, advance the frontiers of computing, and probe the mysteries of life, matter, and the universe. Scientists from around the world rely on the Lab’s facilities for their own discovery science. Berkeley Lab is a multiprogram national laboratory, managed by the University of California for the U.S. Department of Energy’s Office of Science.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit energy.gov/science.